β-Lactams-penicillins, cephalosporins, carbapenems and monobactams
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Mechanisms of action of antibiotics and synthetic anti-infective agents
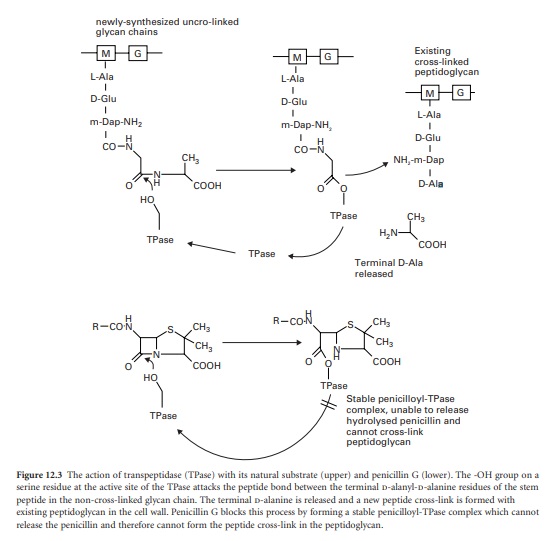
The final stage of peptidoglycan assembly is the cross-linking of the linear glycan strands assembled by trans-glycosylation to the existing peptidoglycan in the cell wall.
β-LACTAMS—PENICILLINS, CEPHALOSPORINS, CARBAPENEMS AND MONOBACTAMS
The final stage of peptidoglycan assembly is the cross-linking of the
linear glycan strands assembled by trans-glycosylation to the existing
peptidoglycan in the cell wall. This reaction is catalysed by transpeptidase
enzymes, which are also located on the outer face of the cell membrane. They
first remove the terminal d-alanine residue from each stem peptide on the newly
synthesized glycan chain. The energy released from breaking the peptide bond
between the two alanines is used in the formation of a new peptide bond between
the remaining D-alanine on the stem peptide and a free amino group present on
the third amino acid of the stem peptides in the existing cross-linked
peptidoglycan. In many organisms, including E. coli, this acceptor amino group
is supplied by the amino acid diaminopimelic acid. In other organisms, e.g.
Staph. aureus, the acceptor amino group is supplied by the amino acid l-lysine.
Although there is considerable variation in the composition of the peptide
cross-link among different species of bacteria, the essential transpeptidation
mechanism is the same. Therefore, virtually all bacteria can be inhibited by
interference with this group of enzymes.
The β-lactam antibiotics inhibit
transpeptidases by acting as alternative substrates. They mimic the
d-alanyl-d-alanine residues and react covalently with the transpeptidases (Figure 12.3).
The β-lactam bond (common to all members of the β-lactam antibiotics) is broken
but the remaining portion of the antibiotic is not released immediately. The
half-life for the transpeptidase-antibiotic complex is of the order of 10
minutes; during this time the enzyme cannot participate in further rounds of
peptidoglycan assembly by reaction with its true sub-strate. The vital
cross-linking of the peptidoglycan is therefore blocked while other aspects of
cell growth continue. The cells become deformed in shape and eventually burst
through the combined action of a weakened cell wall, high internal osmotic
pressure and the uncontrolled activity of autolytic enzymes in the cell wall.
Penicillins, cephalosporins, carbapenems and monobactams all inhibit
peptidoglycan cross-linking through interaction of the common β-lactam ring
with the transpeptidase enzymes. However, there is considerable variation in
the morphological effects of different β-lactams owing to the existence of
several types of transpeptidase. The transpeptidase enzymes are usually
referred to as penicillin binding proteins (PBPs) because they can be separated
and studied after reaction with 14C-labelled penicillin. This step is necessary
because there are very few copies of each enzyme present in a cell. They are
usually separated according to their size by electrophoresis and are numbered
PBP1, PBP2, etc., starting from the highest molecular weight species. In
Gram-negative bacteria most of the high molecular weight transpeptidases also
possess trans-glycosylase activity, i.e. they have a dual function in the final
stages of peptidoglycan synthesis with the trans-glycosylase and transpeptidase
activities located in separate regions of the protein structures. Furthermore,
the different transpeptidases have specialized functions in the cell; all
cross-link peptidoglycan but some are involved with maintenance of cell
integrity, some regulate cell shape and others produce new cross wall between
elongating cells, securing chromosome segregation prior to cell division. The
varying sensitivity of the PBPs towards different β-lactams helps to explain
the range of morphological effects observed in treated bacteria. For example,
penicillin G (benzylpenicillin), ampicillin and cephaloridine are particularly
effective in causing rapid lysis of Gram-negative bacteria such as E. coli.
These antibiotics act primarily upon PBP1B, the major transpeptidase of the
organism. Other β-lactams have little activity against this PBP, e.g.
mecillinam binds preferentially to PBP2 and it produces a pronounced change in
the cells from a rod shape to an oval form. Many of the cephalosporins, e.g.
cephalexin, cefotaxime and ceftazidime, bind to PBP3 resulting in the formation
of elongated, filamentous cells. The lower molecular weight PBPs, 4, 5 and 6,
do not possess transpeptidase activity. These are carboxypeptidases, which
remove the terminal D-alanine from the pentapeptides on the linear glycans in
the cell wall but do not catalyse the cross-linkage. Their role in the cells is
to regulate the degree of cross-linking by denying the d-alanyl-d-alanine
substrate to the transpeptidases but they are not essential for cell growth. Up
to 90% of the amount of antibiotic reacting with the cells may be consumed in
inhibiting the carboxypeptidases, with no lethal consequences to the cells.
Gram-positive bacteria also have multiple transpeptidases, but fewer
than Gram-negatives. Shape changes are less evident than with Gram-negative
rod-shaped organisms. Cell death follows lysis of the cells mediated by the
action of endogenous autolytic enzymes (autolysins) present in the cell wall
which are activated following β-lactam action. Autolytic enzymes able to hydrolyse
peptidoglycan are present in most bacterial walls; they are needed to re-shape
the wall during growth and to aid cell separation during division. Their
activity is regulated by binding to wall components such as the wall and
membrane teichoic acids. When peptidoglycan assembly is disrupted through
β-lactam action, some of the teichoic acids are released from the cells, which
are then susceptible to attack by their own autolysins.
Related Topics
