Advantages and disadvantages using lithium-based drugs
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : Alkali Metals
The name ‘lithium’ stems from the Greek word ‘lithos’ which means stone. Lithium salts are well known for their use in batteries, metal alloys and glass manufacture.
Advantages
and disadvantages using lithium-based drugs
The name ‘lithium’ stems from the Greek word ‘lithos’ which
means stone. Lithium salts are well known for their use in batteries, metal
alloys and glass manufacture. Nevertheless, Li also has a clinical application
in the treatment of manic depression or BD. BD affects 1–2% of the population
and severely reduces the quality of life for the patients and also increases
the likelihood of patients committing suicide. Research has shown that lithium
salts are very successful in the treatment of BD, and a broad research in this
area has been stimulated. The main question addressed within this research is
how the lithium ion (Li+) can be modified in order to improve its
activity to patent their findings, which would result in income for the
manufacturer. Unfortunately, the simple ion is the active ingredient and this
makes it difficult to patent and secure any intellectual property.
Li is a member of group 1 alkali metals and is
the lightest and smallest solid element. Li has an atomic number of 3 and
contains a single valence electron, which determines its redox chemistry and
reactivity. In nature, Li is found as ores, for example, spodumene [LiAl(SiO3)2],
or at low concentrations as salts, for example, in rivers. The metal itself is
soft and white in appearance; it has the lowest reactivity within the group of
alkali metals. The Li+ ion has the smallest ionic radius of all
known metals.
1. Isotopes of lithium and their medicinal application
Lithium occurs as mixture of two stable, naturally occurring
isotopes, namely 6Li with an occurrence of 7.59% and the major
isotope 7Li (92.41%). The nucleus of 6Li contains three
protons and three neutrons, whilst 7Li contains three protons and
four neutrons.
6Li and
7Li are both NMR (nuclear magnetic resonance) active nuclei, which
means their presence can be monitored via NMR technology. Using this analytical
tool, it is possible to differentiate between intra- and extracellular Li+
concentrations and therefore, the uptake of Li+ into different cells
can be monitored. The use of 6Li results in sharper NMR spectra
because of its properties, but it also has a lower intensity. 6Li
salts can also be used to monitor the distribution of lithium in the tissue. A
further application of 6Li is the production of tritium atoms (31H)
and their use in atomic reactors. In this process, 6Li is bombarded
with neutrons, which results in the production of tritium atoms and radioactive
-particles (42He):
63Li+10n→31H+42He
Equation 2.11 shows the activation of 6Li.
2. Historical developments in lithium-based drugs
The first medical use of Li+ was described in 1859
for the treatment of rheumatic conditions and gout. The theory at that time was
based on the ability of lithium to dissolve nitrogen-containing compounds such
as uric acid. Their build-up in the body was believed to cause many illnesses
such as rheumatic conditions and gout problems. In 1880, Li+ was
first reported as being used in the treatment of BD, and in 1885 lithium
carbonate (Li2CO3) and lithium citrate [Li3C3H5O(COO)3]
were included in the British Pharmacopoeia. It also became clear that there is
a direct link between NaCl intake and heart diseases as well as hypertension.
Therefore, LiCl was prescribed as replacement for NaCl in the diet of affected
patients [3b].
The urea hypothesis and the connection of NaCl to heart diseases
stimulated the use of lithium salts in common food. The prime example is the
soft drink 7Up©, which has been marketed in 1929 under the label Bib-Label Lithiated Lemon-Lime Soda. 7Up
contained lithium citrate and was also marketed as a hang-over cure. The actual Li+ was
subsequently removed in 1950 [3b].
John Cade’s experiments on guinea pigs in 1949 initiated the
discovery of Li+ and its sedative and mood-control properties. Uric
acid was known to have mood-controlling properties and Cade used lithium urate
as a control solution. To his surprise, he discovered that lithium urate had
tranquillising properties, and after further experiments he concluded that this
was caused by the lithium ion .
Nevertheless, there were drawbacks, especially when the FDA
banned Li+ salts following the death of four US patients. These
patients had an average intake of 14 g of lithium chloride (LiCl) per day in
order to replace NaCl. Another stumbling stone in the way of success of Li+
was the discovery of chlorpromazine, the first antipsychotic drug, which is
still used for the treatment of BD. In the early 1970s, Li+ was
re-approved by FDA and is now used in 50% of the treatment of BD .
3. The biology of lithium and its medicinal application
Lithium salts are used in the prophylaxis and treatment of
mania, and in the prophylaxis of BD and recur-rent depression. Lithium therapy
is taken orally, usually as lithium carbonate (Li2CO3) or
lithium citrate [Li3C3H5O(COO)3],
with a total dose of up to 30 mmol/day. Li2CO3 is the
preferred lithium salt used, as it causes the least irritation to the stomach.
The treatment has to be closely monitored, and Li+ blood
concentra-tions are measured 12 h after administration to achieve a serum
lithium concentration of 0.4–1 mmol/l. The therapeutic index (concentration
window from efficacy to toxicity) for Li+ is very narrow, and plasma
Li+ concentrations above 2 mM require emergency treatment for
poisoning (Figure 2.6) .
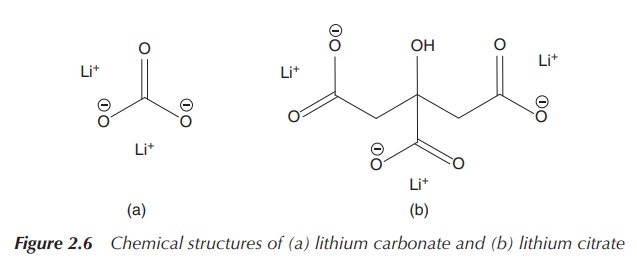
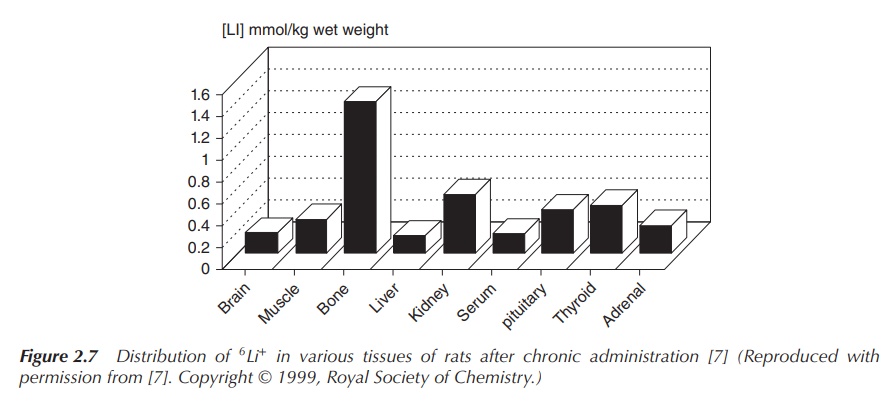
The administered Li+ is distributed uniformly in the
body tissues and in the blood plasma, with the external cell Li+
concentration being below 2 mM. Experiments studying the lithium distribution
in rats after admin-istration of a high dose of 6Li+
showed that there was no exceptional accumulation of 6Li+
in the brain. 6Li+ distributes fairly uniformly in the
body, with bones and endocrine glands showing higher concentrations (Figure
2.7) .
Li+ ions are not soluble in lipids
and therefore do not cross plasma membranes. The transport into cells occurs
via exchange mechanism by lithium–sodium counter-transport, anion exchange
(so-called Li+/CO32− co-transport) and other
unrelated transport molecules. The specific mode of action of the simple Li+
ion is currently unknown, but it is clear that a displacement of Mg2+
by Li+ is involved. Therefore, an alteration of the Mg2+
balance in the blood and the urine can be observed in patients treated with Li+
[3b]. This displace-ment is actually not surprising because the properties of
Li+ and Mg2+ are similar, which can be explained by the
concept of diagonal relationship.
4. Excursus: diagonal relationship and periodicity
Within the periodic table, the element pairs
Li/Mg, Be/Al, B/Si and others form a so-called diagonal rela-tionship to each other. With this concept,
similarities in biological activity can be explained as the element pairs have similar properties.
Diagonally
adjacent elements of the second and third periods have similar properties –
this concept is called diagonal
relationship. These pairs (Li/Mg, Be/Al, B/Si, etc.) have similarities in
ion size, atomic radius, reactive behaviour and other properties.
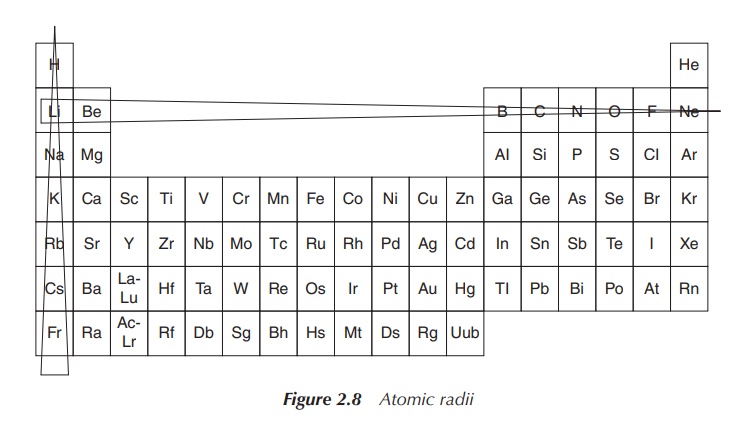
Diagonal relationship is a result of opposite effects, which
crossing and descending within the periodic table has. The size of an atom
decreases within the same period (from left to right). The reason is that a
positive charge is added to the nucleus together with an extra electron
orbital. Increasing the nucleus charge means that the electron orbitals are
pulled closer to the nucleus. The atomic radius increases when descending
within the same group, which is due to the fact that extra orbitals with
electrons are added (Figure 2.8) .
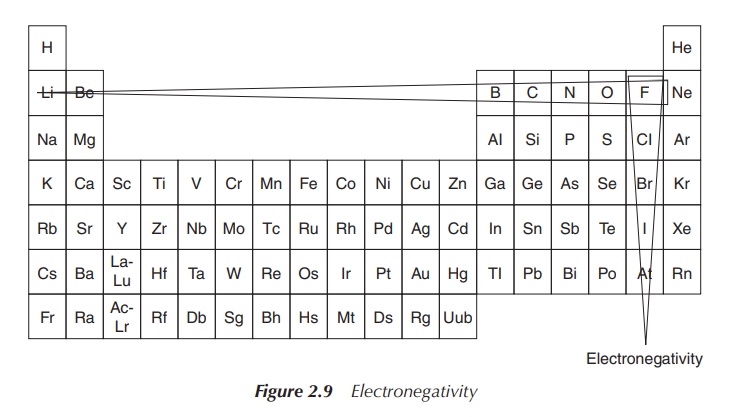
Trends can be seen for the electronegativity and the ionisation
energy; both increase when moving within the same period from left to right and
decrease within the same group. Within a small atom, the electrons are located
close to the nucleus and held tightly, which leads to a high ionisation energy.
Therefore, the ionisation energy decreases within the group and increases
within the period (from left to right) – showing the opposite trend compared to
the atomic radius. A similar explanation can also be used for summarising the
trends seen for the electronegativity: small atoms tend to attract electrons
more strongly than larger ones. This means fluorine is the most electronegative
element within the Periodic Tables of Elements (Figure 2.9).
This structure of trends is summarised under the term periodicity of the elements. This means
that within the periodic table, all elements follow the above-mentioned trends
in a repetitive way. This allows predicting trends for atomic and ionic radii,
electronegativity and ionisation energy (Figure 2.10).
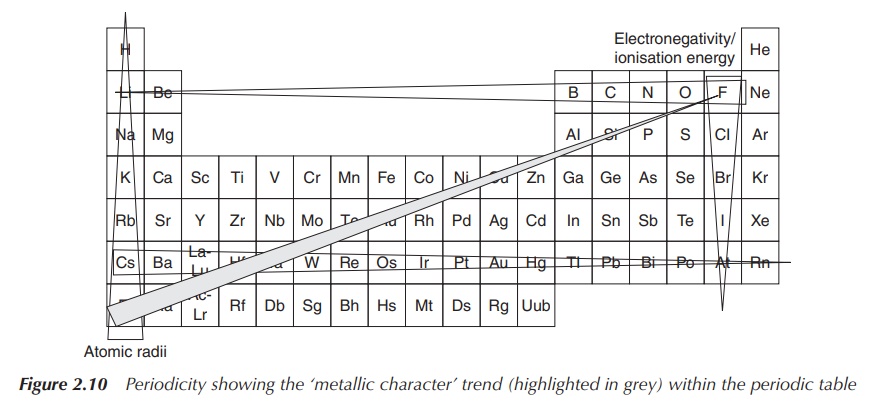
In relation to the diagonal relationship, it becomes clear by studying the described trends that these effects cancel each other when descending within the group and crossing by one element within the PSE. Therefore, elements diagonally positioned within the periodic table have similar properties, such as similar atomic size, electronegativity and ionisation energy (Figure 2.11).
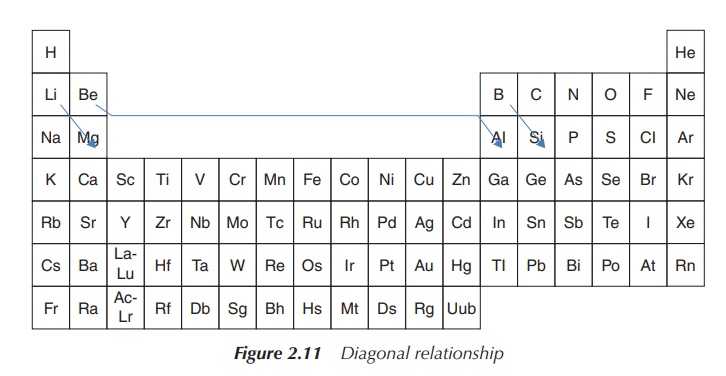
The concept of the diagonal relationship is crucial for the
biological activity of lithium drugs, which is mainly due to the properties of
the Li+ ion being similar to the Mg2+ ion. In comparison,
the size of the Li+ ion is similar to that of Mg2+ and
therefore they compete for the same binding sites in proteins. Nevertheless,
lithium has relatively specific effects, and so only proteins with a low
affinity for Mg2+ are targeted. Li+ and Mg2+
salts have similar solubility, for example, CO32−, PO43−,
F− salts have a low water solubility, and halide and alkyl salts are
soluble in organic solvents. Li+ and Mg2+ compounds are
generally hydrated, for example, LiCl⋅3H2O and MgCl2⋅6H2O. Similarities in ionic size,
solubility, electronegativity and solubility result in similar biological
activity and therefore pharmaceutical application [3b].
5. What are the pharmacological targets of lithium?
The precise mechanism of action of lithium ions as mood
stabilisers is unknown. Current research shows that there are two main targets
in the cell – the enzymes glycogen synthase kinase-3 (GSK-3) and the
phospho-monoesterases family (PMEs) [3b].
GSK-3 is a serine/threonine protein kinase, which is known to
play an important role in many biological processes. GSK-3 is an enzyme that
mediates the addition of phosphate molecules onto the hydroxyl groups of
certain serine and threonine amino acids, in particular cellular substrates. Li+
inhibits GSK-3 enzymes via competition for Mg2+ binding .
Phosphoric monoester hydrolases (PMEs) are enzymes that catalyse
the hydrolysis of O—P bonds by nucleophilic attack of phosphorous by cysteine
residues or coordinated metal ions. Inositol monophosphatase (InsP) is the best
known member of this family. Li+ and Mg2+ both have a
high affinity to bind to phos-phate groups. Li+ inhibits the
enzymatic function of InsP and prevents phosphate release from the active site.
Generally, inositol phosphatases are Mg2+-dependent, but Li+
binds to one of the catalytic Mg2+ sites. Binding Li+ to
phosphate-containing messenger molecules could perturb the transcellular
communication and thus be antipsychotic [3b].
Lithium inhibits GSK-3 and InsP, and both pathways have
therefore been suggested to be involved in the treatment of BD and
schizophrenia. The theory behind this hypothesis is that overactive InsP
signalling in the brain of these patients potentially causes BD and this may be
reduced by the inhibitory effect of lithium on such signalling .
GSK-3 alters structure of cerebella neurons, and lithium is a
neuroprotective agent that can reduce the hypersensitivity to toxins as seen in
cells that overexpress GSK-3. It is believed that lithium potentially can
protect against disease-induced cell death. GSK-3 has been implicated in the
origins of schizophrenia, but with the availability of many antipsychotic drugs
on the market, lithium ions are not in common use for the treatment of
schizophrenia .
There are also several direct roles of lithium in the treatment of
Alzheimer’s disease. Alzheimer’s disease is a neurodegenerative brain disorder
causing neuronal dysfunction and ultimately cell death. This leads ulti-mately
to dementia, affecting in the United States around 10% of people aged over 65
and 48% aged over 85. Onset occurs with the accumulation of extracellular
senile plaques composed of amyloid-β peptides and with the accumulation of
intercellular neurofibrillary tangles. GSK-3 is necessary for the accumulation
of tangles, and subsequently GSK-3 inhibition reduces the production of
peptides – this is where the Li+ interaction comes into play [3b].
6. Adverse effects and toxicity
Lithium has a very narrow therapeutic window, which makes the
monitoring of blood levels essential during the treatment. Blood plasma
concentrations of more than 1.5 mmol/l Li+ may cause toxic effects,
usually tremors in the fingers, renal impairment and convulsion. Also, memory
problems are a very common side effect, and complaints about slowed mental
ability and forgetfulness are commonly reported. Extreme doses of lithium can
cause nausea and diarrhea, and doses above 2 mmol/l require emergency
treat-ment, as these levels may be fatal. Weight gain and decreased thyroid
levels are also commonly reported problems. The blood serum level of Li+
has to be monitored 12 h after administration, and health care pro-fessionals
also have to be aware that the mood-stabilising effects of lithium take a
couple of days to take effect .
Lithium salts have severe adverse effects on the renal system.
Lithium therapy can damage the internal structures of the kidneys, which are
the tubular structure of the nephrons, and can lead to diabetes insipidus. One
out of five patients experience polyuria – excess production of urine, which is
the number one symptom of diabetes insipidus. Therefore, the kidney function
has to be closely monitored for patients undergoing long-term therapy, with a
full test of the kidney function every 6–12 months for stabilised regimes [3a,
6].
Great care has to be taken when lithium is given with
nonsteroidal anti-inflammatory drugs (NSAIDs) because up to 60% increase of Li+
concentration in blood has been observed. NSAIDs reduce the clearance of
lithium through kidneys, and as a result lithium poisoning is possible.
Furthermore, the concurrent use of diuretics, which can result in sodium
depletion, can make lithium toxicity worse and can be hazardous [3a].
Related Topics
