Aldehydes
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Functional Group Synthesis
The aldehyde functional group is a very reactive functional group; thus methods to prepare it must be mild and allow the aldehyde group to survive the reaction conditions.
ALDEHYDES
The
aldehyde functional group is a very reactive functional group; thus methods to
prepare it must be mild and allow the aldehyde group to survive the reaction
conditions. Traditional methods for introduction of the aldehyde functional
group include
Aldehydes
are intermediate in oxidation level, and thus the aldehyde func-tional group
can be installed by either reduction of carboxylic acid derivatives or
oxidation of alcohols. Aldehydes are rarely installed without a change of
oxi-dation level. One difficulty is that they undergo both oxidation and reduction readily. Special methods
are required to stop at the aldehyde stage rather than proceeding by further
reduction or oxidation.
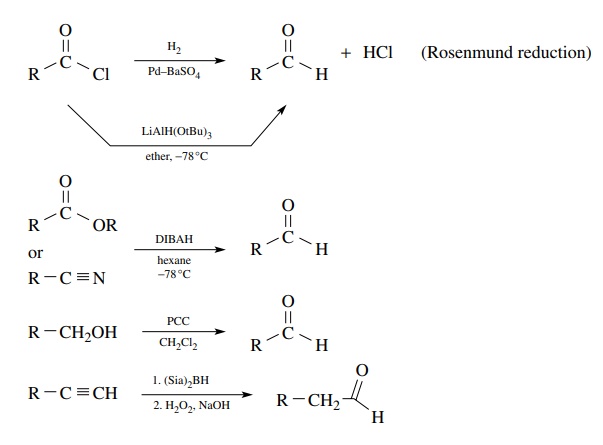
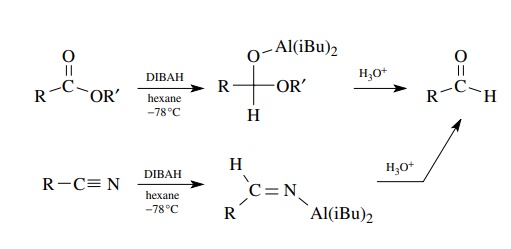
Reductive
methods utilize carboxylic acid derivatives as starting materials, and the
trick is to stop the reduction at the aldehyde stage, which is normally more
easily reduced than the starting material. While there are a variety of
reducing systems known and many employ acid chlorides as precursors, the most
effective reduction method for the preparation of aldehydes is the diisobutylaluminum
hydride (DIBAH) reduction of either esters or nitriles using a single
equivalent of the reducing agent. By using low temperatures, the intermediate
anions produced by hydride addition are at the aldehyde oxidation level, but
they are resistant to further reduction. Hydrolysis delivers the aldehyde. Care
must be taken to maintain low temperature during both the reaction and the
hydrolysis.
The
oxidation of primary alcohols to aldehydes also suffers from the problem of overoxidation
of the aldehyde to a carboxylic acid. Mild methods capable of stopping the
oxidation at the aldehyde oxidation level are required if aldehydes are to be
obtained. The most common and effective reagent for this purpose is pyridinium
chlorochromate (PCC), produced by the reaction of pyridinium hydrochloride with
chromium trioxide. This reagent is soluble in dichloromethane and smoothly
oxidizes primary alcohols to aldehydes in high yields. Because of the mild,
neutral reaction conditions and the use of stoichiometric amounts of oxidant,
the aldehyde product is not oxidized further.
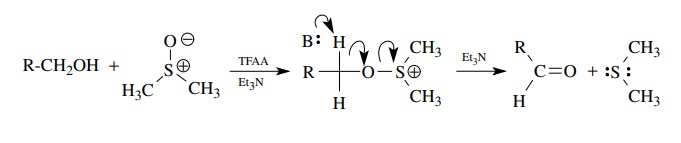
The
activation of DMSO by electrophilic reagents such as oxallyl chloride or
trifluoroacetic anhydride (TFAA) (among many others) produces an oxidant
capable of oxidizing primary alcohols to aldehydes in high yields. This
oxidation is called the Swern oxidation and yields the aldehyde (oxidized
product) by reductive elimination of dimethylsulfide (reduced product) and
proceeds under mild, slightly basic conditions. It is a second widely used and
effective oxidative method for the production of aldehydes from primary
alcohols.
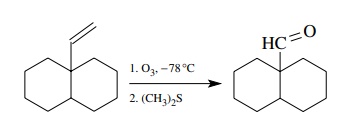
A
different oxidative approach toward the preparation of aldehydes uses the
ozonolysis of vinyl groups. If a vinyl group is present in a molecule, it can
be oxidatively cleaved to an aldehyde by ozonolysis. This process cleaves the
carbon – carbon double bond, but it is mild and very successful in many cases.
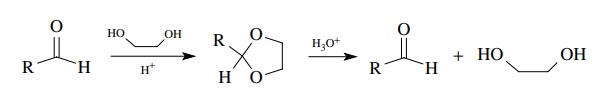
The
formation of aldehydes without a change in oxidation level is not a common
synthetic approach because most compounds that can be hydrolyzed to aldehydes
without change in the oxidation level are formed from aldehy-des in the first
place. Thus acetals can be hydrolyzed rapidly to aldehydes by acidic water, but
they are normally prepared from aldehydes. As such this is a very common
protection strategy for aldehydes wherein they are first con-verted to an
acetal and later hydrolyzed back to the aldehyde when the time is right.