What is organometallic chemistry?
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : Organometallic Chemistry
Organometallic chemistry is the area of chemistry that deals with compounds containing a metal–carbon bond. As such, this area combines aspects from both organic and inorganic chemistry.
What
is organometallic chemistry?
Organometallic chemistry is the area of
chemistry that deals with compounds containing a metal–carbon bond. As such,
this area combines aspects from both organic and inorganic chemistry.
An organometallic compound is
characterised by the presence of one or more carbon–metal bonds.
It is important to note that the metal can
either be a member of the s or p block on one hand or a d-block metal
(transition metal) on the other. There are no real direct pharmaceutical
applications known for group 1 and 2 organometallic compounds, as they are very
reactive reagents, except that they are commonly involved in the synthesis of
modern medicines. Examples of such synthetic reagents include sodium
cyclopentadienide (NaCp, NaC5H5) and butyl lithium (BuLi)
compounds.
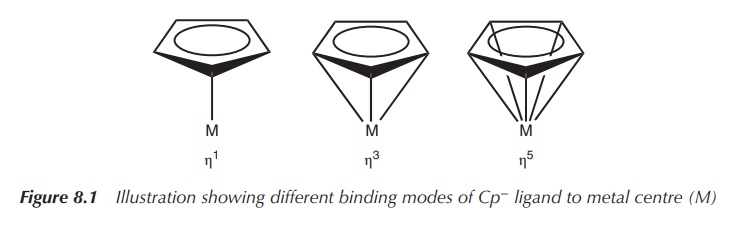
Cyclopentadienyl or Cp− (C5H5−) is a commonly used ligand in organometallic
chemistry, which is ver-satile in
the number of bonds it can form to a metal centre (M). There are different ways
of representing these interactions (see
also Figure 8.1).
NaCp is an organometallic agent that is mainly used to introduce
a cyclopentadienyl anion (C5H5−) to a metal
centre in order to form a so-called metallocene (see Chapter 8 section 2 for a
definition of metallocenes). NaCp can be synthesised by the reaction of either
sodium with cyclopentadiene or from dicyclopentadiene under heating. Sodium
hydride (NaH) can also be used as a base, instead of sodium, to deprotonate the
acidic CH2 group of the cyclopentadiene (Figure 8.2).
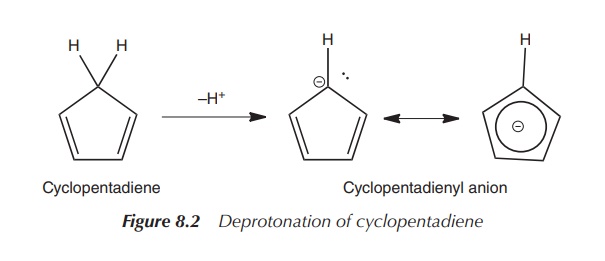
It is interesting to look at the bonding in this Cp ligand. First of all, it is important to understand the formation of the cyclopentadienyl anion, the Cp− ligand. Cyclopentadiene is surprisingly acidic, which is a result of the resonance stabilisation of the resulting cyclopentadienyl anion (Cp−).
The cyclopentadienyl anion
follows Huckel’s rule (4n + 2, n = 0, 1, 2, etc.), which means that the
Cp− anion is a planar carbocycle of aromatic nature.
An aromatic molecule has to be a cyclic
and planar molecule with an uninterrupted network of electrons.
Furthermore, it has to fulfil the Huckel rule, which states it has (4n + 2) electrons.
Organolithium compounds (Li—C bond) are probably the best known
organometallic agents. In organic synthesis and drug design, they can be used
as either an extremely potent base or as nucleophile; in the latter case, the
organic moiety will be introduced to the target molecule. They are typically
synthesised by reacting an organohalide (RX) with elemental lithium. The best
known examples are n-butyllithium (n-BuLi), sec-butyllithium (s-BuLi)
and tert-butyllithium (tert-BuLi), with tert-BuLi being the most reactive (Figure 8.3).
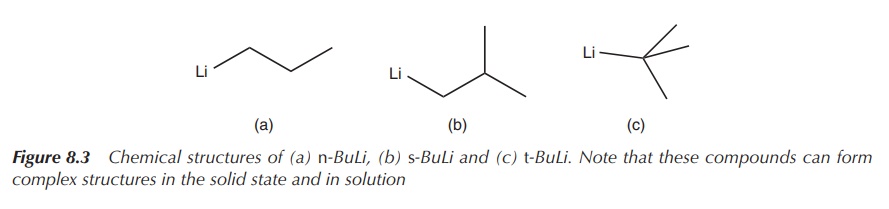
Figure 8.3 Chemical structures of (a) n-BuLi, (b) s-BuLi and (c) t-BuLi. Note that these compounds can form complex structures in the solid state and in solution
Alkali metal organometallics are more or less pyrophoric, which means they combust spontaneously on contact with air. They have to be handled under the exclusion of air, humidity and oxygen and are mostly stored in hydrocarbons. The solvent plays an important role and can be responsible for potential decomposition processes or an increased or reduced reactivity.
The heteroatoms
of solvents can potentially also coordinate to the alkali metal and therefore
influence potential cluster formation of the organometallic compound. Alkali
metal organometallics are known to form relatively complex clusters in solution
and in the solid state, which influences their reactivity.
Organometallic compounds, containing d-block metals, are
currently under intense research within the pharmaceutical chemistry area in
order to find new treatment options for cancer and diabetes, amongst others.
d-Block organometallics are generally fairly stable complexes, which in
contrast to alkali metal organometallics can be handled in the presence of air.
Whilst s- and p-block organometallics form and bonds between the metal and the
organic group, in d-block organometallics the number of bonds, which is called hapticity, can be further increased.
The most common ligands for d-block organometallics include
carbon monoxide (CO) in the form of the carbonyl group, phosphanes (PR2H)
and derivatives of the cyclopentadienyl (Cp−) ligand. A
characteristic example is the bonding of the metal with the carbonyl ligand,
which can be described as one M—CO interac-tion. A vacant (hybridised) orbital
of the metal centre forms a bond with the CO ligand, which means that
electronic charge is donated from the CO ligand to the metal centre. As CO is
also a π-acceptor ligand, a back donation of electronic charge from the metal
centre can occur. This donation/back donation interplay results in a
strengthening of the metal–carbon bond and a weakening of the carbon–oxygen
bond. CO is classified as a σ-donor and π-acceptor molecule.
The chemistry of d-block organometallic chemistry covers a vast
amount of material and therefore we will concentrate on the area of so-called
metallocenes, which are complexes containing typically a d-block metal and two
Cp− ligands. This area encompasses the most promising drug-like
candidates so far.
Related Topics
