Phosphorus
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : Group 15 Elements
Phosphorus (P) is a nonmetal of the nitrogen group. As previously outlined, +3 and +5 are the preferred oxi-dation states, forming a variety of allotropes, with black phosphorus being the most stable one.
Phosphorus
Phosphorus (P) is a nonmetal of the nitrogen
group. As previously outlined, +3 and +5 are the preferred oxi-dation states,
forming a variety of allotropes, with black phosphorus being the most stable
one. Phosphorus is one of the most abundant elements in the human body and is
often found in conjunction with calcium, because together they are the building
materials for bones and teeth. Phosphorus is also involved in the building of
our genetic material as well as in the energy supply of cells and many
biochemical processes.
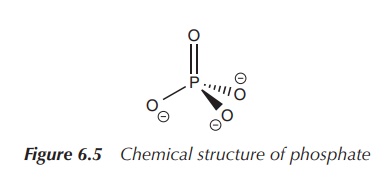
Within the human body, phosphate is the main
phosphorus-containing compound. Phosphate is an inorganic compound and is the
salt of phosphoric acid. It can form organic esters with a variety of compounds
and these are important in many biochemical processes. Phosphate has the
empirical formula PO43−. It is a tetrahedral molecule,
where the central phosphorus atom is surrounded by four oxygen atoms (Figure
6.5).
The phosphate ion PO43−
is the conjugated base of the hydrogen phosphate ion (HPO42−).
HPO42− is the conjugated base of the dihydrogen phosphate
ion (H2PO4−). The latter is the conjugated
base of phosphoric acid (H3PO4).
A conjugated base is formed from an acid
by the removal of a proton. This means that the conjugate base of an acid is
this acid without a proton. An analogous definition applies to a conjugate
acid.
A conjugate base (of the acid) + H+ → Acid
A conjugate acid (of the base) → Base
+ H+
In biological systems, phosphate is often
found either as the free ion (inorganic phosphate) or as an ester after
reaction with organic compounds (often referred to as organic phosphates). Inorganic phosphate (mostly denoted as Pi)
is a mixture of HPO42− and H2PO4−
at physiological pH.
Adenosine phosphates: ATP, ADP and AMP
Adenosine phosphates are organic-phosphate-containing compounds
that are responsible for the energy flow in many biochemical processes in
living cells. Adenosine phosphates consist of three parts: a sugar molecule
(ribose) as the backbone, to which a nucleobase adenine and a varying number of
phosphate groups are con-nected. Adenine is bonded to C-1 of the sugar, whilst
the phosphate groups are connected to each other and then are attached to the
C-5 atom of the ribose backbone. There is a series of adenosine phosphates
depend-ing on the number of phosphate groups present. Adenosine triphosphate
(ATP) contains three phosphate groups, whilst adenosine diphosphate (ADP)
contains two and adenosine monophosphate (AMP) contains one phosphate group
(Figure 6.6) .
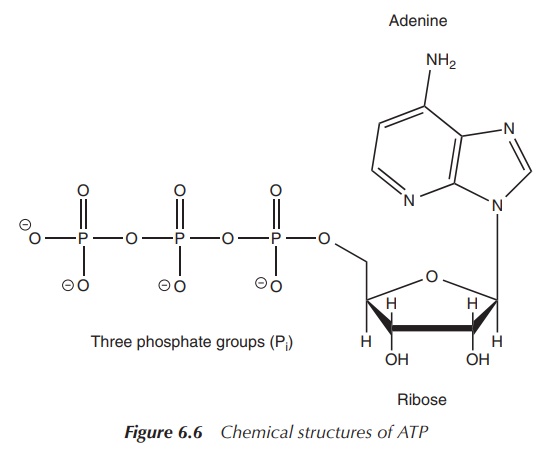
Within living cells, energy is transferred by dephosphorylation
of ATP, which results in a transfer of energy to biochemical processes and the
production of ADP. The enzyme ATPase is used to cleave off the phosphate group.
Phosphate in DNA
DNA is a major macromolecule in all living
organism responsible for the encoding of genetic material. Mostly, DNA consists
of a double-stranded helix. Each strand has a backbone of alternating sugar
moieties (deoxyri-bose) and phosphate groups. The phosphate group is attached
to 5′ position of the deoxyribose (Figure 6.7).
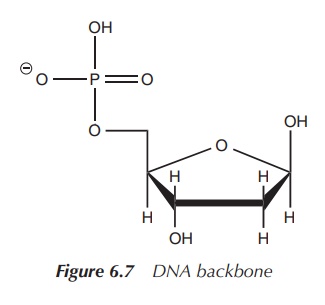
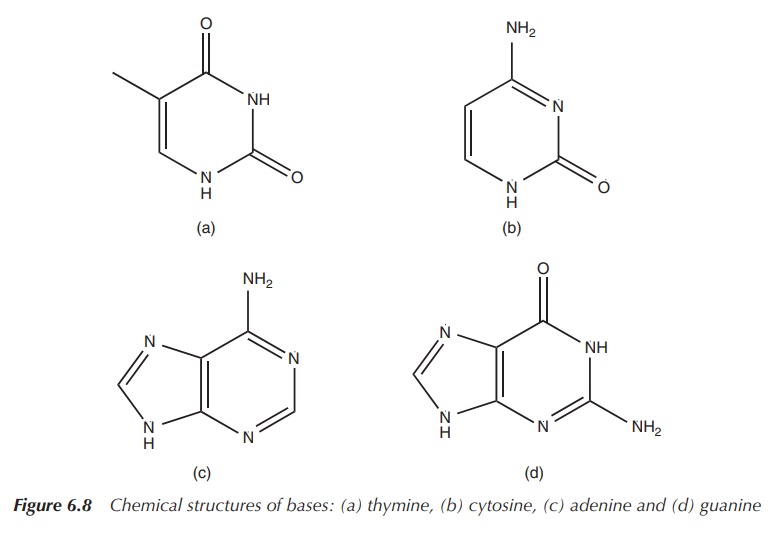
There is also an organic base attached to the sugar moiety in
the 1′ position. In DNA, these organic bases are thymine (T),
cytosine (C), adenine (A) or guanine (G) (Figure 6.8). This unit, the
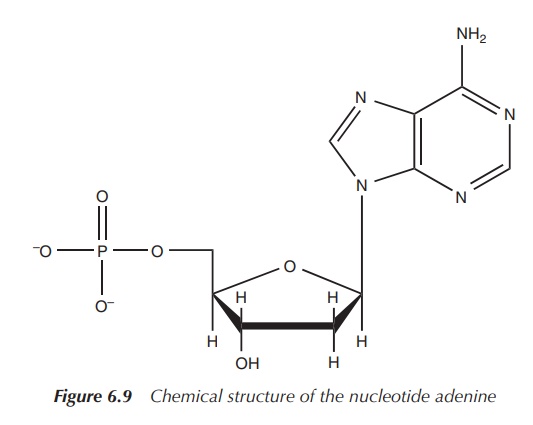
deoxyribose with the phosphate group and a base, is called a nucleotide (Figure 6.9).
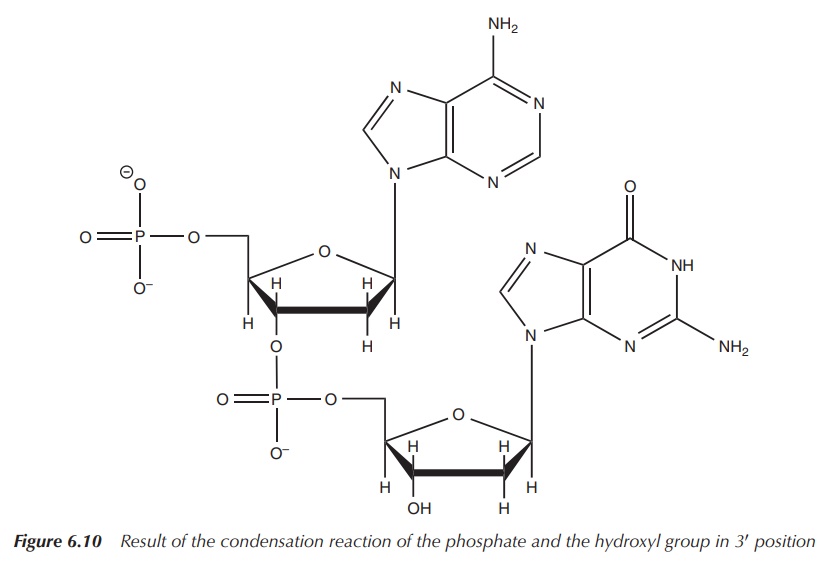
These nucleotides are then joined together by a condensation
reaction of the phosphate and the hydroxyl group in 3′ position
(Figure 6.10).
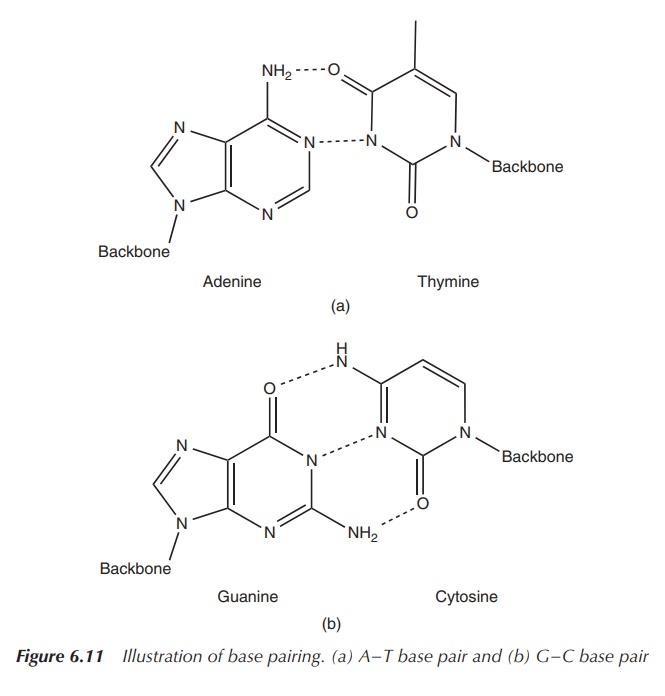
As previously mentioned, DNA mostly exists as a double-stranded
helix. The double strand is formed by the interaction between the base pairs.
Adenine and thymine form two hydrogen bonds, whereas guanine and cytosine are
held together by three hydrogen interactions (Figure 6.11).
It is important to note that the two strands run in opposite
directions. Whilst one strand runs in the 5′ –3′
direction, the other one runs in the 3′ –5′ direction.
Clinical use of phosphate
Phosphorus-containing compounds, mainly phosphates, are usually
present in abundance in the human diet.
They are mostly found in milk, meat (protein-rich food), grains, dried fruits and carbonated soft drinks.
Hypophosphataemia (low levels of phosphate in the serum) is rare and is often caused by some underlying illness, extreme lifestyle situations such as starvation or alcoholism or drug interactions; for example, some diuretics may cause low phosphate levels. Hyperphosphataemia, in contrast, is more common and often caused by kidney problems (reduced clearance) or dietary behaviour (increased intake). Phosphate and calcium ions work closely together and therefore an imbalance of either ion can have serious consequences for bone health or can even lead to cardiovascular problems due to hardening of the soft tissue [2, 3].
The recommended daily allowance for dietary phosphate ranges
between 700 and 1250 mg depending the circumstances, and typically no
supplementation in healthy humans is necessary . Phosphate supple-mentation
should be taken only under medical supervision and is usually indicated in the
following cases: Hypophosphataemia, hypercalcaemia (high levels of blood Ca2+
levels) and sometimes for calcium-based kidney stones. Oral phosphate
supplementation will be needed only in a minority of patients, often in
patients dependent on alcohol or with severe underlying medical conditions.
Oral phosphate tablets and solutions typically contain a mixture of monobasic
sodium phosphate, sodium dihydrogen phosphate and/or disodium phosphate.
Intravenous (IV) preparations containing phosphate together with potassium and
sodium ions can be used in extreme cases of hypophosphataemia .
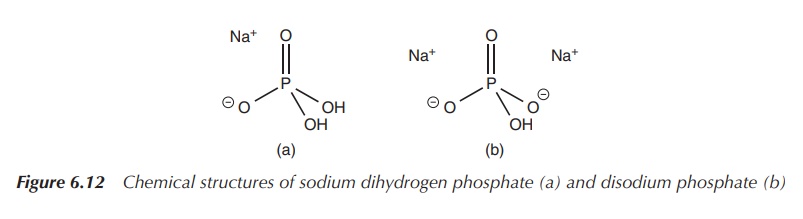
Phosphate solutions can also be used in enemas, where they
display their laxative properties. Phosphate enemas are used for the clearance
of the bowel before any surgery or endoscopy. A typical phosphate enema
solution contains a mixture of sodium dihydrogen phosphate and hydrated
disodium phosphate (Figure 6.12) .
Hyperphosphataemia is a more common problem seen in patients either because of excessive intake of phosphate or owing to reduced renal clearance such as in patients with renal failure. In this case, phosphate binding agents are used to treat the patients to manage high blood phosphate levels. Mostly, calcium prepa-rations (e.g. calcium citrate tablets or capsules) are used as phosphate binding agents. The aim is to bind any excess phosphate in the gut before absorption. In patients on dialysis, Sevelamer or lanthanum salts (see Chapter 11) can be used to maintain normal phosphate blood levels. Sevelamer is a polymer containing amine groups. These protonated amine groups can react with the negatively charged phosphate groups via ionic bind-ing and prevent absorption of phosphate from the gut. In the past, aluminium preparations were used, but they are not recommended anymore because of potential aluminium accumulation (see Chapter 4) .
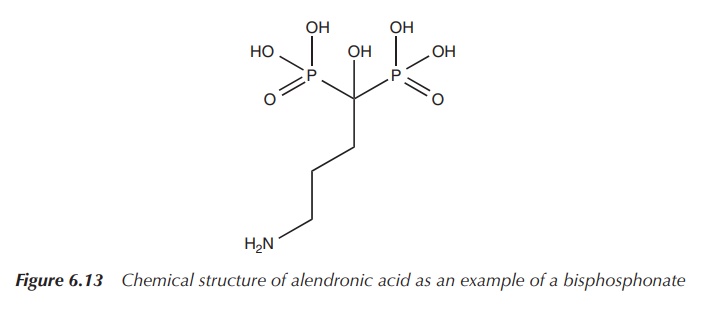
Bisphosphonates are structural analogues to pyrophosphate and
contain two phosphate groups linked together by a central carbon centre
containing an organic side chain. The phosphonate is absorbed onto the
hydroxyapatite crystals in the bone, thereby slowing down any metabolic
processes in the bone. The side chain influences the skeletal binding and
prevents the enzymatic breakdown in the gastrointestinal tract by phosphatases.
Bisphosphonates are mainly licensed for the treatment of osteoporosis, and alendronic acid is mostly the drug of choice. Administration typically is by mouth as tablets once a day or once a week for post-menopausal women .
It is interesting to note that the oral bioavailability
of bisphosphonates, which are typically large hydrophilic molecules, is very
low with only up to 1.5% of the administered dose . Bisphosphonates are large
hydrophilic molecules, which prevents their diffusion through the transcellular
route . Additionally, the negative charges hinder other absorption mechanisms
and encourage reaction with positively charged ions and molecules (Figure
6.13).
Bisphosphonates can also be used for the treatment of
hypercalcaemia and pain from metastatic bone cancer in patients with breast
cancer. The treatment with bisphosphonates can have severe side effects,
ranging from gastrointestinal disturbances to osteonecrosis of the jaw .
Drug interactions and toxicity
Excess of phosphate can lead to interactions within the human
body with calcium, iron and magnesium, and can lead to diarrhoea and may even
be toxic. Phosphate and calcium levels are directly connected, and an excess of
phosphate will lead to the removal of calcium from the bones and teeth. This
will cause osteo-porosis and problems with the health of teeth and gums.
Athletes often use phosphate supplementation, but a healthcare specialist
should monitor this application.
Interactions for phosphate preparations with several
over-the-counter and prescription drugs are known. Antacids containing
aluminium, calcium and magnesium ions can bind phosphate in the digestive tract
and prevent phosphate from being absorbed. This can lead in extreme cases to
hypophosphataemia. Potassium-sparing diuretics and potassium supplements in
combination with phosphate preparations may lead to elevated levels of blood
potassium levels (hyperkalaemia). Hyperkalaemia can be a serious
life-threatening problem (see Chapter 2).
Related Topics
