Aseptic Areas
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Principles Of Good Manufacturing Practice
The operative is a potential source of microorganisms and it is imperative that steps are taken to prevent this contamination.
ASEPTIC AREAS
A) Additional Requirements
i) Clothing
The operative is a potential
source of microorganisms and
it is imperative that steps
are taken to prevent this contamination. The operative
must wear sterile protective headwear
totally enclosing hair and beard,
spectacles, powder-free rubber
or plastic gloves (often two pairs are worn), a non-fibre-shedding
facemask (to prevent the release
of droplets) and footwear. A suitable
garment is a one
or two-piece trouser suit. Fresh sterile
clothing should be provided each time
a person
enters an aseptic
area.
ii) Entry to aseptic areas
Entry to an aseptic
suite is usually
through a ‘black–grey– white’ changing
procedure (Figure 23.4),
where white represents the highest
level of cleanliness. Movement from ‘black’ to ‘white’ is via two changing rooms,
the ‘grey’ area also serving as an entry
to the cleanroom (Figure 23.4). There are several types
of entry system
in use. More details
may be found in Whyte
(2010).
iii)
Equipment and operation
Any articles entering the aseptic area should ideally
be sterilized, but may be disinfected. In order to achieve this, articles should be transferred via a double-ended sterilizer or hatch
(i.e. with a door
at each end).
If they are not
to be discharged directly to the aseptic
area, they should be (1) double-wrapped before
sterilization; (2) transferred immediately after sterilizing into a clean environment until required; (3) transferred from this clean environment via a double-doored hatch (where the outer
wrapping is removed) to the aseptic
area (where the inner wrapper is removed at the workbench). Hatches and sterilizers must be designed so that only
one door may be
opened at any one time.
Solutions manufactured in the cleanroom may be brought
into the aseptic
area through a sterile 0.22 μm membrane
filter.
Workbenches,
including laminar flow
units, and equipment, should
be disinfected immediately before and after each work session. Equipment must be of the simplest design possible for the operation
being performed. Aseptic manipulations must be carried out in the grade A air of a laminar
flow cabinet or isolator. Speed, accuracy and economy of movement are essential features
of good aseptic
technique. It is therefore essential that workers
are well trained
and motivated and familiar
with
the task in hand. Observation and microbiological monitoring of the operator and of the environment are very important. Under no circumstances must living microorganisms, including those used for vaccine preparation and
for biological monitoring be introduced into the
aseptic area.
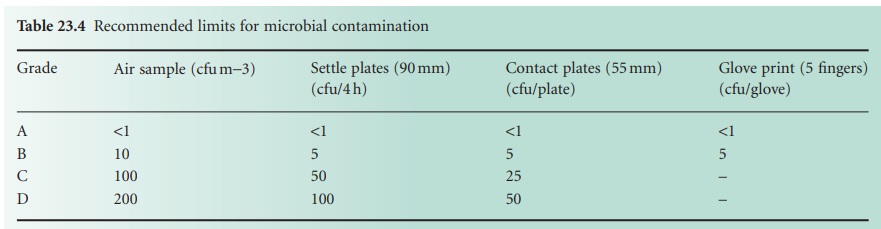
B) Environmental Monitoring
Monitoring of the environment is essential during
manufacturing. It ensures
that environmental requirements are being met and also helps spot trends.
Air is monitored for
particles and microorganisms.
Microorganisms are usually sought using settle plates
or active samplers, such as the slit-toagar sampler.
Settle plates rely on organisms falling from the atmosphere and
settling onto an exposed agar
plate. After a specified time (usually 4 hours) the plate will be
covered and incubated. A slit-to-agar sampler
draws in a specified volume of air, forcing organisms onto the surface of an agar plate.
This latter method
is able to give
a viable
count per volume,
but organisms may
be damaged and hence rendered
non-viable by the capture process. Limits of viable
counts for different
grades of air are
shown in Table 23.4. One of the
limitations of traditional microbial detection is the time
taken to culture
bacteria and fungi. There
is a great deal of interest in developing
rapid (Denyer, 2007) or instantaneous (Jiang, 2009) methods
of microbial detection.
The nature
of contamination can be informative. For example, the presence of Staphylococcus
spp. suggests human-borne contamination. The adequacy of changing facilities and gowning
would then be checked. In contrast, Bacillus
spores would suggest environmental contamination and the entry of equipment into the
cleanroom would be scrutinized.
Glove prints are taken by applying four
fingers and a thumb
to an agar plate. This ensures that disinfection of gloves is adequate. Surfaces
may be monitored by swapping or by using contact
plates. The latter
have the advantage of providing
a quantitative measure
of surface contamination,
but there is a risk of leaving
agar deposits on the surface
(Butson & Hawitt,
2008).
C) Eliminating Human Intervention
The greatest source of contamination in the cleanroom comes from the operating staff (Whyte & Hejab, 2007). Movement of staff can increase
particle shedding and disrupting laminar airflow.
It is not surprising, therefore, that modern practices seek to minimize
or even eliminate humans from the aseptic production area. This can be
achieved by the use of automation, of isolators and of
restricted access
barriers (RABs).
All aseptic
packaging should be carried out
in a grade A environment with
a grade B background (Table 23.2). Advances in technology now permit
the production of self-contained workstations, or isolators, which incorporate many of the design principles of cleanrooms
and laminar
flow cabinets.
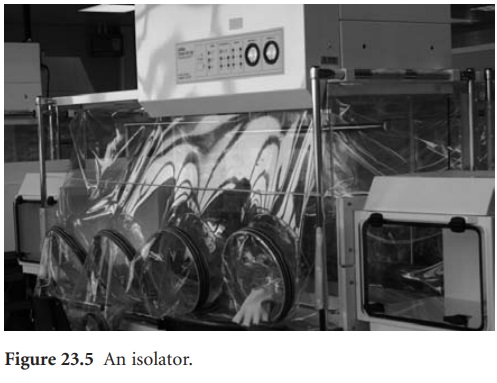
i) Isolators
The isolator both protects the product from contamination by the operator and the operator from any hazardous materials. Direct interaction between
the operator and the product is minimized by providing
a grade A laminar flow
of air with
a positive pressure, the internal space
being accessed by means of a glove/ sleeve system (Figure
23.5). A grade
D background is considered adequate for such
operations. A fuller
account of isolators
is given by Midcalf et al. (2004).
ii)
Restricted
access barrier systems
Restricted access
barrier systems (RABS) provide a level of control intermediate between an isolator
and a cleanroom (Agalloco & Akers, 2006).
They allow for easier
intervention than an isolator but require a grade B background.
iii)
Blow–fill–seal technology
Blow–fill–seal units
are purpose-built pieces of equipment which
carry out these
three steps in a continuous process within a controlled environment. Containers are formed from thermoplastic granules and blown
to form containers which
are then filled
and heat-sealed. These units are fitted with
a grade A air shower
and operated in a grade C environment for aseptic manufacture and a grade D background for products which
are to be terminally sterilized.
Related Topics
