Atomic Structure
| Home | | Anatomy and Physiology | | Anatomy and Physiology Health Education (APHE) |Chapter: Anatomy and Physiology for Health Professionals: Levels of Organization : Chemical Basics of Life
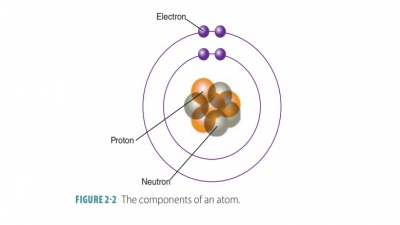
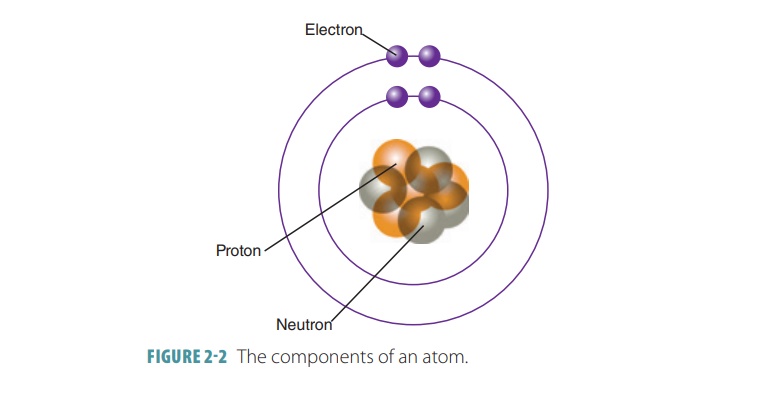
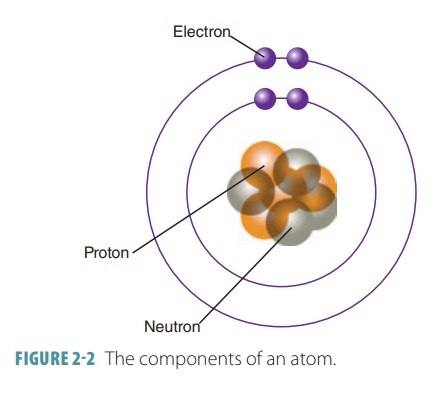
Atoms are composed of subatomic particles and each atom consists of protons, neutrons, and electrons.
Atomic
Structure
Atoms are composed of subatomic
particles and each atom consists of protons, neutrons, and electrons. Protons
and neutrons are similar in size and mass; however, protons bear a positive electrical charge, whereas neutrons are electrically neutral (uncharged). Electrons bear a negative electrical charge. An atom’s mass is determined mostly
by the number of protons and neutrons in its nucleus. The nucleus contains approximately the entire mass (99.9%) of the
atom. The mass of a larger object, such as the human body, is the sum of the
masses of all its atoms. FIGURE 2-2 shows the components of an atom and its nucleus.
Electrons orbit an atom’s nucleus
at high speed, forming a spherical electron cloud. Atoms normally contain equal numbers of protons and electrons.
The number of protons in an atom is known as its atomic
number. Thus, hydrogen (H),
the simplestatom, has one proton, giving it the atomic number 1, whereas
magnesium, with 12 protons, has the atomic number 12.
The atomic
weight of an element’s atom equals the
number of protons and neutrons in its nucleus. For example, oxygen has eight
protons and eight neutrons, so its atomic weight is 16. An isotope is
defined as when an element’s atoms have nuclei containing the same number of
protons but different number of neu-trons. Isotopes may or may not be
radioactive. Radio-activity is the emission of energetic particles known as radiation, which occurs because of
instability of the atomic nuclei.
The nuclei of certain isotopes (radioisotopes)
spontaneously emit subatomic particles or radiation in measurable amounts. The
process of emitting radi-ation is called radioactive
decay. Strong radioactive isotopes are dangerous because their emissions
can destroy molecules, cells, and living tissue. For diag-nostic procedures,
weak radioactive isotopes are used to diagnose structural and functional
characteristics of internal organs. Radiation is basically identified as one of
three common forms: alpha (α), beta (β), or gamma (γ). Gamma radiation is the
most penetrating type and is similar to X-ray radiation.
Health professionals and researchers use radioac-tive isotopes for clinical applications because they are easily detected and measured. All isotopes of a certain element have the same atomic number. For example, two types of iodine, 125-iodine and 131-iodine, can substitute for 126-iodine in chemical reactions. Iodine may be used in diagnostic procedures involving the thyroid gland to detect thyroid cancer.
1. Define
the term “atom” and explain its structure.
2. Differentiate
between atomic weight and atomic number.
3. Describe
the locations of electrons, protons, and neutrons.
4. Define
the terms “atomic number” and “radioisotopes.”