Binding of Drugs to Blood Components
| Home | | Biopharmaceutics and Pharmacokinetics |Chapter: Biopharmaceutics and Pharmacokinetics : Protein Binding of Drugs
Following entry of a drug into the systemic circulation, the first things with which it can interact are blood components like plasma proteins, blood cells and haemoglobin.
BINDING OF DRUGS TO BLOOD COMPONENTS
Plasma Protein-Drug Binding
Following entry of a drug into the systemic
circulation, the first things with which it can interact are blood components
like plasma proteins, blood cells and haemoglobin (see Table 4.1). The main interaction of drug in the blood
compartment is with the plasma proteins which are present in abundant amounts
and in large variety. The binding of drugs to plasma proteins is reversible.
The extent or order of binding of drugs to various plasma proteins is:
Abumin > α1 Acid Glycoprotein >
Lipoproteins > Globulins.
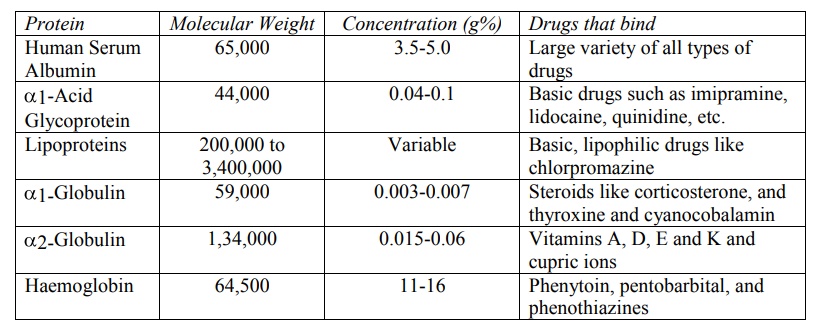
TABLE 4.1
Blood Proteins to which Drugs Bind
Binding of Drugs to Human Serum Albumin
The human serum albumin (HSA), having a molecular
weight of 65,000, is the most abundant plasma protein (59% of total plasma and
3.5 to 5.0 g%) with a large drug binding capacity. The therapeutic doses of
most drugs are relatively much smaller and their plasma concentration do not
normally reach equimolar concentration with HSA. The HSA can bind several
compounds having varied structures. Both endogenous compounds such as fatty
acids, bilirubin and tryptophan as well as drugs bind to HSA. A large variety
of drugs ranging from weak acids, neutral compounds to weak bases bind to HSA.
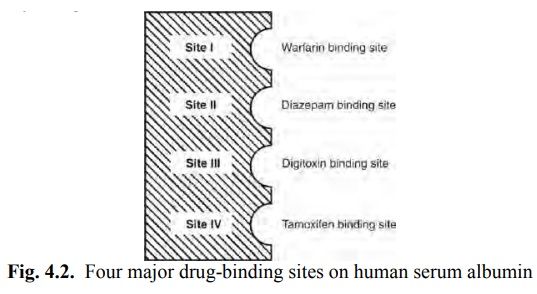
Four different sites on HSA have been identified for drug-binding (Fig. 4.2).
They are:
Site I: Also called as warfarin and azapropazone binding site, it represents the region to which large number of drugs are
bound, e.g. several NSAIDs (phenylbutazone, naproxen, indomethacin),
sulphonamides (sulphadimethoxine, sulphamethizole), phenytoin, sodium valproate
and bilirubin.
Site II: It is also called as the diazepam binding site. Drugs which bind to this region include
benzodiazepines, medium chain fatty acids, ibuprofen, ketoprofen, tryptophan,
cloxacillin, probenicid, etc.
Site I and site II are responsible for the binding
of most drugs.
Site III: is also called as digitoxin binding site.
Site IV: is also called as tamoxifen binding site.
Very few drugs bind to sites III and IV.
A drug can bind to more than one site in which case
the main binding site is called as the primary
site and the other as the secondary
site; for example, site I is the primary site for dicoumarol and site II the secondary site. Groups of drugs that
bind to the same site compete with each other for binding, but drugs that bind
to one site do not competitively inhibit binding of drugs to other sites.
However, they may either promote or retard binding of a drug to another site by
energetic coupling mechanisms.
Binding of Drugs to α1-Acid Glycoprotein (α1-AGP or AAG)
Also called as the orosomucoid, it has a molecular weight of 44,000 and a plasma
concentration range of 0.04 to 0.1 g%. It binds to a number of basic drugs like
imipramine, amitriptyline, nortriptyline, lidocaine, propranolol, quinidine and
disopyramide.
Binding of Drugs to Lipoproteins
Binding of drugs to HSA and AAG involve hydrophobic
bonds. Since only lipophilic drugs can undergo hydrophobic bonding,
lipoproteins can also bind to such drugs because of their high lipid content.
However, the plasma concentration of lipoproteins is much less in comparison to
HSA and AAG.
A drug that binds to lipoproteins does so by
dissolving in the lipid core of the protein and thus its capacity to bind
depends upon its lipid content. The molecular weight of lipoproteins varies
from 2 lakhs to 34 lakhs depending on their chemical composition. They are
classified on the basis of their density into 4 categories –
1. Chylomicrons (least dense and
largest in size).
2. Very low density lipoproteins
(VLDL).
3. Low-density lipoproteins (LDL)
(predominant in humans).
4. High-density lipoproteins
(HDL) (most dense and smallest in size).
The hydrophobic lipid core of these macromolecules
consists of triglycerides and cholesteryl esters and the relatively hydrophilic
surface is made of apoproteins (free cholesterol and proteins). Predictably,
VLDL is rich in triglycerides and HDL is rich in apoproteins.
Binding of drugs to lipoproteins is non-competitive
i.e. there are no specific or non-specific binding sites and binding is not
dependent on drug concentration. Binding rather reflects partitioning of drugs
in hydrophobic core of lipoprotein molecule. A number of acidic (diclofenac),
neutral (cyclosporin A) and basic drugs (chlorpromazine) bind to lipoproteins.
Basic, lipophilic drugs have relatively more affinity. Lipoprotein binding
becomes significant in cases of drugs that predominantly bind to them, and
secondly, when levels of HSA and AAG in plasma are decreased.
The main physiological role of lipoproteins is
circulation of lipids to tissues through the blood. Similarly, lipoproteins
also play an important role in the transport of drugs to tissues.
Binding of Drugs to Globulins
Several plasma globulins have been identified and
are labelled as α1-, α 2-, β1-, β2- and γ- globulins.
1. α1-globulin: also called as transcortin or
CBG (corticosteroid binding globulin), it binds a number of steroidal drugs
such as cortisone and prednisone. It also binds to thyroxine and
cyanocobalamin.
2. α 2-globulin: also called as ceruloplasmin,
it binds vitamins A, D, E and K and cupric
ions.
3. β 1-globulin: also called as transferrin,
it binds to ferrous ions.
4. β 2-globulin: binds to carotinoids.
5. γ-globulin: binds specifically to antigens.
Binding of Drugs to Blood Cells
More than 40% of the blood comprises of blood cells
of which the major cell component is the RBC. The RBCs constitute 95% of the
total blood cells. Thus, significant RBC drug binding is possible. The red cell
is 500 times in diameter as the major plasma protein binding component,
albumin. The RBC comprises of 3 components each of which can bind to drugs:
1. Haemoglobin: It has a molecular weight of
64,500 (almost equal to that of HSA) but
is 7 to 8 times the concentration of albumin in blood. Drugs like
phenytoin, pentobarbital and phenothiazines bind to haemoglobin.
2. Carbonic Anhydrase: Drugs
known to bind to it are acetazolamide and
chlorthalidone (i.e. carbonic anhydrase inhibitors).
3. Cell Membrane: Imipramine and chlorpromazine are
reported to bind with the RBC membrane.
It has been shown that the rate and extent of entry
into RBC is more for lipophilic drugs, e.g. phenytoin. Hydrophilic drugs like
ampicillin do not enter RBC.
Related Topics
