Biopharmaceutics and Pharmacokinetics
| Home | | Biopharmaceutics and Pharmacokinetics |Chapter: Biopharmaceutics and Pharmacokinetics : Introduction
Drugs, whether obtained from plant, animal or mineral sources or synthesized chemically, are rarely administered in their pure chemical form.
Biopharmaceutics and Pharmacokinetics
Introduction
Drugs, whether obtained from plant, animal or mineral sources or synthesized chemically, are rarely administered in their pure chemical form. Often, they are combined with a number of inert substances (excipients/adjuvants) and transformed into a convenient dosage form that can be administered by a suitable route. Earlier, it was believed that the therapeutic response to a drug is an attribute of its intrinsic pharmacological activity. But today, it is very much understood that the dose-response relationship obtained after drug administration by different routes—for example, oral and parenteral, are not the same. Variations are also observed when the same drug is administered as different dosage forms or similar dosage forms produced by different manufacturers, which in turn depend upon the physicochemical properties of the drug, the excipients present in the dosage form, the method of formulation and the manner of administration. A new and separate discipline called biopharmaceutics has therefore been developed to account for all such factors that influence the therapeutic effectiveness of a drug.
Biopharmaceutics is defined as the study of factors influencing the rate and amount of drug that reaches the systemic circulation and the use of this information to optimise the therapeutic efficacy of the drug products. The process of movement of drug from its site of administration to the systemic circulation is called as absorption. The concentration of drug in plasma and hence the onset of action, and the intensity and duration of response depend upon the bioavailability of drug from its dosage form. Bioavailability is defined as the rate and extent (amount) of drug absorption. Any alteration in the drug’s bioavailability is reflected in its pharmacological effects. Other processes that play a role in the therapeutic activity of a drug are distribution and elimination. Together, they are known as drug disposition. The movement of drug between one compartment and the other (generally blood and the extravascular tissues) is referred to as drug distribution. Since the site of action is usually located in the extravascular tissues, the onset, intensity and sometimes duration of action depend upon the distribution behaviour of the drug. The magnitude (intensity) and the duration of action depend largely upon the effective concentration and the time period for which this concentration is maintained at the site of action which in turn depend upon the elimination processes. Elimination is defined as the process that tends to remove the drug from the body and terminate its action. Elimination occurs by two processes— biotransformation (metabolism), which usually inactivates the drug, and excretion which is responsible for the exit of drug/metabolites from the body.
In order to administer drugs optimally, knowledge is needed not only of the mechanisms of drug absorption, distribution, metabolism and excretion (ADME) but also of the rate (kinetics) at which they occur i.e. pharmacokinetics. Pharmacokinetics is defined as the study of time course of drug ADME and their relationship with its therapeutic and toxic effects of the drug. Simply speaking, pharmacokinetics is the kinetics of ADME or KADME. The use of pharmacokinetic principles in optimising the drug dosage to suit individual patient needs and achieving maximum therapeutic utility is called as clinical pharmacokinetics. Figure 1.1 is a schematic representation of processes comprising the pharmacokinetics of a drug.
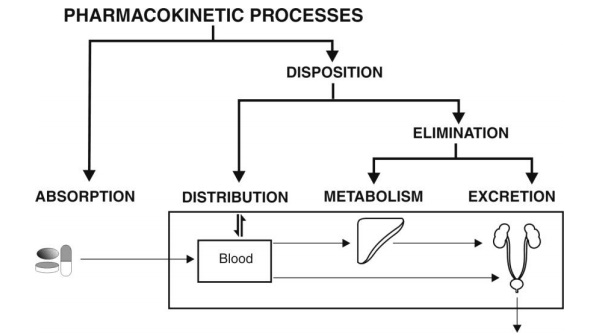
Fig. 1.1. Schematic illustration of pharmacokinetic processes
Drug administration and therapy can now be conveniently divided into four phases or processes:
1. The Pharmaceutical Phase: It is concerned with –
(a) Physicochemical properties of the drug, and
(b) Design and manufacture of an effective drug product for administration by a suitable route.
2. The Pharmacokinetic Phase: It is concerned with the ADME of drugs as elicited by the plasma drug concentration-time profile and its relationship with the dose, dosage form and frequency and route of administration. In short, it is the sum of all the processes inflicted by the body on the drug.
3. The Pharmacodynamic Phase: It is concerned with the biochemical and physiologic effects of the drug and its mechanism of action. It is characterized by the concentration of drug at the site of action and its relation to the magnitude of effects observed. Thus, in comparison –
Pharmacokinetics is a study of what the body does to the drug, whereas Pharmacodynamics is a study of what the drug does to the body.
Pharmacokinetics relates changes in concentration of drug within the body with time after its administration, whereas
Pharmacodynamics relates response to concentration of drug in the body.
4. The Therapeutic Phase: It is concerned with the translation of pharmacological effect into clinical benefit.
A schematic representation of the various processes involved in the therapy with a drug is given in Fig. 1.2.
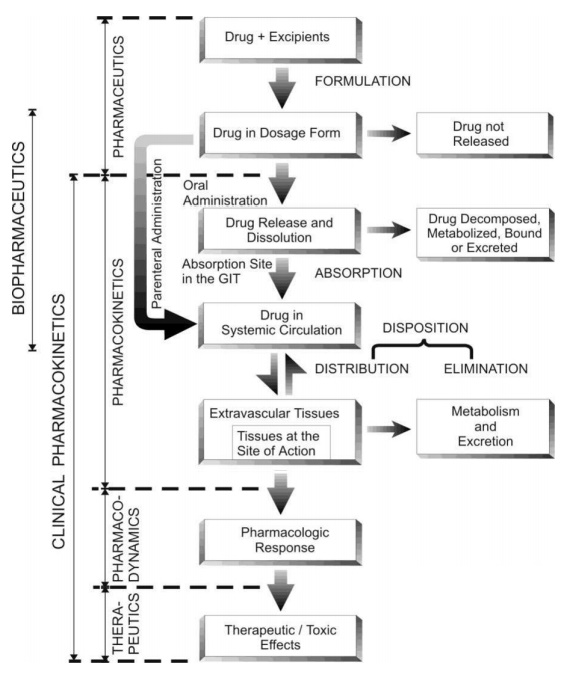
Fig. 1.2. Schematic representation of the processes involved in drug therapeutics
To achieve optimal therapy with a drug, the drug product must be designed to deliver the active principle at an optimal rate and amount, depending upon the patient’s needs. Knowledge of the factors affecting the bioavailability of drug helps in designing such an optimum formulation and saves many drugs that may be discarded as useless. On the other hand, rational use of the drug or the therapeutic objective can only be achieved through a better understanding of pharmacokinetics (in addition to pharmacodynamics of the drug), which helps in designing a proper dosage regimen (the manner in which the drug should be taken). This obviates the use of the empirical approach where a considerable experimentation is needed to arrive at the balance between the desired therapeutic and the undesired toxic effects in order to define an appropriate dosage regimen.
The knowledge and concepts of biopharmaceutics and pharmacokinetics thus have an integral role in the design and development of new drugs and their dosage forms and improvement of therapeutic efficacy of existing drugs.
Related Topics
