Chemistry of group 15 elements
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : Group 15 Elements
Nitrogen makes up 78% (by volume) of air, whereas phosphorus can be found in several minerals and ores.
Chemistry
of group 15 elements
Occurrence and extraction
Nitrogen makes up 78% (by volume) of air, whereas phosphorus can
be found in several minerals and ores. Phosphorus is an essential constituent
of plants and animals, being present in deoxyribonucleic acid (DNA), bones,
teeth and other components of high biological importance. Phosphorus does not
occur in its elemental state in nature, as it readily oxidises and therefore is
deposited as phosphate rock. The remaining elements of group 15 are mostly
obtained from minerals, but can also be found in their elemental form in the
earth’s crust. Arsenic is mostly presented in nature as mispickel (FeAsS),
realgar (As4S4) and orpiment (As2S3).
Bismuth occurs as bismuthinite (Bi2S3) as well as in its
elemental form.
Dinitrogen (N2) is extracted by fractional distillation of liquid air. By-products such as dioxygen (O2) are removed by addition of H2 and the use of a Pt catalyst. Elemental phosphorus is extracted from phosphate rock by reacting with sand and coke in an electrically heated industrial oven. Phosphorus vapour is isolated and condensed under water – white phosphorus is extracted.
2Ca3(PO4)2 + 6SiO2 +
10C → P4
+ 6CaSiO3 + 10CO
Elemental arsenic is extracted mainly from FeAsS by heating and
subsequent condensation of arsenic in the absence of air.
FeAsS → FeS + As (in absence of air)
Antimony is obtained from stibnite (Sb2S3)
after reduction with iron. Bismuth is extracted from its sulfide or oxide ores
via a reduction with carbon.
Sb2S3 + 3Fe → 2Sb + 3FeS
Physical properties
The physical properties of group 15 elements vary widely, from
nitrogen being a gas to the remaining elements being solids with increasing
metallic character. Nitrogen exists as a diatomic molecule N2 and is
a colourless and odourless gas (condensation at 77 K). Nitrogen forms
relatively strong and short bonds, resulting in the formation of a triple bond
in the N2 molecule. Furthermore, nitrogen has an anomalously small
covalent radius and therefore can form multiple bonds with N, C and O atoms.
Group 15 elements follow the general trend showing an increasing covalent
radius when descending within the group.
Phosphorus has several allotropes, with white,
red and black phosphorus being the main ones.
Allotropes are defined as the two or more physical forms of one element.
Allotropes of carbon are graphite, carbon
and diamond. These allotropes are all based on carbon atoms but exhibit different
physical prop-erties, especially with regard to hardness.
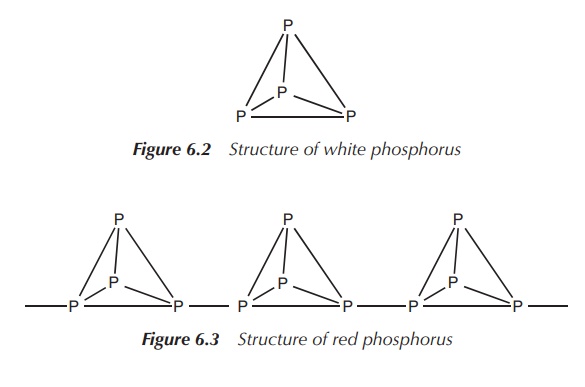
White phosphorus is a solid consisting of
tetrahedral P4 molecules with single bonds. White phospho-rus is the
standard state of the element, but it is metastable, potentially due to the
strained 60∘ bond
angles (Figure 6.2).
Heating of white phosphorus in an inert gas atmosphere results
in red phosphorus, which is an amorphous solid (several crystalline forms are
known) with an extended covalent structure (Figure 6.3).
Black phosphorus is the most stable allotrope of phosphorus and
can be obtained by heating white phospho-rus under high pressure. In contrast
to white phosphorus, black phosphorus does not ignite spontaneously in air. The
reactivity of red phosphorus lies between those of the white and black
allotropes. White phosphorus is insoluble in water and is therefore stored
under water to prevent oxidation.
Arsenic and antimony vapour consists of As4 or Sb4
molecules, respectively. In the solid state, arsenic, antimony and bismuth are
grey solids with a lattice structure similar to that of black phosphorus.
Oxidation states and ionisation energy
The general electron configuration for group 15 elements is ns2np3 and all elements form the oxidation states of +3 and
+5. Nitrogen is more versatile and shows a range of oxidation states ranging
from −3 to +5.
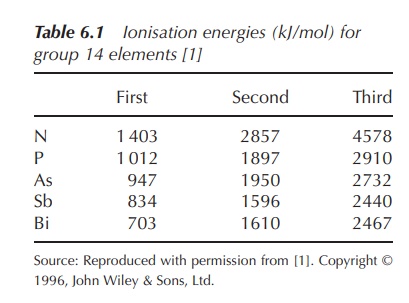
The ionisation energies relating to the
removal of the first five electrons (two s and three p electrons) are
relatively low. There is a significant increase in the ionisation energy
necessary for the removal of a sixth electron, as this will be removed from an
inner complete quantum shell (Table 6.1).
Chemical properties
Nitrogen is relatively unreactive because the bond enthalpy of
the nitrogen–nitrogen triple bond is very high (944 kJ/mol). N2(g)
is usually used as an inert atmosphere for reactions that cannot be carried out
in oxy-gen. Only lithium reacts directly with nitrogen with the formation of Li3N.
Nitrogen fixation is an important
mechanism developed by some microorganism in order to directly incorporate
nitrogen gas into proteins. This process is an important step in the early food
chain.
There are five oxides
of nitrogen known – N2O, NO, N2O3, NO2
and N2O5 (oxidation numbers ranging from +1 to +5,
respectively). Nitric(III) acid (nitrous acid) HONO and nitric(V) acid (nitric
acid) HNO3 are the most important oxoacids of nitrogen. HNO3
is a highly reactive oxidising and nitrating agent.
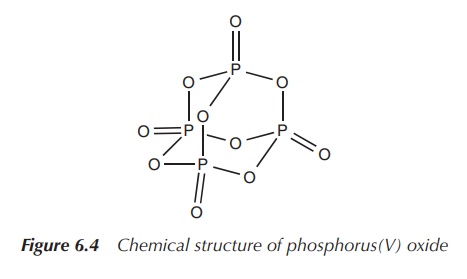
In general, phosphorus is more reactive than nitrogen. White
phosphorus ignites spontaneously in air and forms phosphorus(V) oxide.
Phosphoric acid (H3PO4) is the most important oxoacid of
phosphorus and its main use is in the manufacture of fertilisers (Figure 6.4).
Hydrogen-containing compounds of nitrogen and phosphorus, namely
NH3 and PH3, both act as a Lewis base because of their
lone pair. Phosphine (PH3) is less water soluble than NH3
as it does not form hydrogen bonds. NH3 (ammonia) is produced in the
so-called Haber Bosch process. This industrial process uses finely divided iron
as catalyst and a reaction temperature of around 450 ∘C at a pressure of 50
atm. Ammonia is used to produce fertilisers, nitric acid, nylon and many more
products important to our modern life style.
N2(g) + 3H2(g) → 2NH3(g)
For clinical applications, nitrogen and phosphorus compounds are
mostly used as heteroatoms in organic compounds or counter-ions in inorganic
salts with no specific therapeutic effect. Arsenic differs because it exhibits
its own typical therapeutic and toxic properties, which has resulted in its
long-standing use in clinical applications and in the invention of
chemotherapy. Therefore, the following clinical discussion will concentrate on
arsenic-based drugs.
Related Topics
