Clinical governance and risk management
| Home | | Hospital pharmacy |Chapter: Hospital pharmacy : Medicines information
Clinical governance, with its focus on quality, is an essential element of MI practice. The concept of clinical governance was introduced into the NHS by A First Class Service: Quality in the New NHS.
Clinical governance and risk management
Clinical governance,
with its focus on quality, is an essential element of MI practice. The concept
of clinical governance was introduced into the NHS by A First Class Service:
Quality in the New NHS. Subsequently, the Royal Pharmaceutical Society
published a framework for clinical governance in pharmacy. It identified four
main components for achieving excellence:
· clear lines of responsibility and accountability for overall
quality of clinical care
· a comprehensive programme of quality improvement activities
(including audit, continuing professional development, research and
development)
· clear policies aimed at managing risks
· procedures for identification of poor performance.
This approach has
been reinforced following various reviews where quality of healthcare has been
seen to fall below acceptable standards.
Table 8.4 Legal and ethical issues in medicines information (MI)
Issue Details
Negligence and liability
An MI
pharmacist has a duty to ensure that all information and advice supplied is as
accurate and comprehensive as could reasonably be expected. If that information
or advice, when acted on, causes loss or damage to a patient, the MI pharmacist
may be liable in negligence. For the MI pharmacist to be shown to be negligent
it must be established that the MI pharmacist had a duty of care towards the
patient, that the duty of care was breached and that damage to the patient
occurred. It is, therefore, incumbent on an MI pharmacist to keep up to date as
far as is reasonable with current developments and knowledge, to use all
reasonably available resources to provide the information required, to present
that information in a usable and intelligible form and to act in a professional
manner which is appropriate to the skills possessed and the service offered by
an MI pharmacist. Working to defined standards with agreed minimum resources
and complying with standards for safe systems of work and documentation are
part of this process
Unlicensed and clinical trial medicines
MI
pharmacists can provide information and advice about unlicensed medicines or
unlicensed uses of medicines as long as the enquirer or user is clearly
informed that this is the case
Proactive information
The
same principles apply to written proactive information, for example, that
supplied in bulletins or new-product evaluations. MI pharmacists should be able
to demonstrate the process undertaken to produce the information. Disclaimers,
although bringing to the attention of the information users their
responsibilities in using that information, do not negate the liability of the
MI pharmacist supplying the information. These issues apply to both hard copy
and electronically published information
Defamation
MI pharmacists have a duty when providing information to ensure that information is accurate, fair and produced from demonstrable and quality evidence. Failure to do so, leading to unreasonable loss of commercial success of a medicine, could lead to the pursuance of defamation of product by a pharmaceutical company. However, a genuine error or omission would not normally be grounds for such an action
The UKMi network has
implemented a comprehensive clinical governance programme and utilises a range
of tools to support this programme. The tools are common to other areas of
healthcare provision and include defined national practice standards, quality
assurance programmes, training and competency frameworks. UKMi has also
developed a research strategy.
Standards
National standards
for MI services were first introduced in 1990. Since then they have been
revised and expanded to cover all of the principal elements of the service. In
defining the standards, account is taken of identified best practice, policy
and regulatory developments, technology advancements and the requirements of
commissioners, stakeholders and other organisations with which MI work. The
current standards cover six core areas relevant to most MI centres and a
seventh that is specific to those centres providing a national specialist
service.
The first standard
states that an MI centre must have appropriate space, facilities and resources
to ensure the provision of a safe and efficient service. The elements of this
standard include staffing levels, the working environment and the availability
of appropriate equipment, facilities and information resources. Information
resources that are considered to be essential to local and regional/national MI
centres to enable them to provide a safe and robust enquiry-answering service
are defined.
Provision of the
enquiry-answering service has four associated stan-dards. Achievement of these
standards requires that the service is easily accessible and organised to allow
prompt handling of enquiries, that it meets quantifiable and consistent
criteria to measure user satisfaction and that it meets quantifiable and
consistent criteria for assessing the quality of enquiry answers. The latter
includes assessment of the analysis of the enquiry, the utilisation and
interpretation of appropriate resources and the construction and delivery of an
appropriate, timely and evidence-based answer that incorporates relevant
practical, clinical advice and is tailored to a specific patient.
A further standard
for enquiry-answering, and for the production of pro-active information, for
example bulletins and guidelines, is adequate docu-mentation of the process and
adequate procedures for record management. These have always been important for
provision of an efficient MI service to prevent duplication of work and allow
information to be retrieved if follow-up is required (for example, complaint or
legal case). However, with the call for greater transparency in
decision-making, the implementation of the Freedom of Information Act and the
introduction of the NHS information governance framework these are now
imperative. The experience MI staff have developed in this area puts them in an
excellent position to act as knowledge managers for the pharmacy department,
advising on best practice for documentation and information storage.
The standard for
publications and proactive work includes other elements relating to text
production, proofreading and accuracy checking, and adher-ence to legal
requirements such as copyright. The training standard covers the requirements
for training of MI staff and training provided within MI centres. MI staff
deliver a significant amount of training to other members of the pharmacy
service, providing them with skills relating to problem-solving and critical
appraisal that are transferable to all aspects of professional practice.
A relatively new
standard relates to research and service development. It requires the manager
of an MI service to develop the service and demonstrate its value through
participation in audit, practice research and other similar activities. The
standard reflects the launch of the UKMi national research strategy in 2006
with the aim of driving innovation, improving service quality and sharing good
practice, as well as providing data relating to the impact of MI on the wider
organisation.
Risk management
The sixth standard
relates to risk management, although this plays a major element in all the
other standards. Hospital trusts have wider risk management programmes with
which MI services will comply. In addition, MI centres in the UK should develop
their own risk management plans, reviewed annually, to provide a framework for
safe working. A risk management-based approach to quality-assuring written
information has been suggested to address this approach, utilising broad risk
management techniques.
As part of the risk
management standard, MI centres must have a specified set of standard operating
procedures (SOPs). A number of these have been produced nationally for local
adaptation. They are available on the UKMi website (www.ukmi.nhs.uk). This
portfolio includes SOPs on handling and documenting enquiries, dealing with
enquiries in the absence of an MI pharmacist, dealing with difficult callers
and enquiries that might be consid-ered for onward referral and adverse
incident reporting.
An important
component of clinical governance is learning from experi-ence, both of success
and failure. MI has established an incident-reporting scheme to record errors
and near-misses occurring within the service, similar to those in place for
dispensing and aseptic manufacture. The Incident Reporting in Medicines Information
System (IRMIS) was introduced in 2005 and is held on a secure web-based
database hosted by NHSnet. The scheme is intended to complement existing
reporting schemes within NHS organisations and users are asked to report any
incidents to their trust system in addition to IRMIS. The aim of the system is
to enable the network to collate data on anonymous reports, identify common
themes and look at ways to avoid future incidents. Quarterly reports are
distributed to all MI staff and are used as a learning tool within individual
centres, at local meetings and on national training courses. Recurrent
incidents include confusion over drug names and calculation errors. The system
has also identified errors within manufacturers’ information and published
resources. These have been incorp-orated into UKMi guidance on risk issues with
commonly used MI sources.
Monitoring standards
MI centres are
recommended to undertake regular peer review sessions. These focus principally
on enquiry-answering and provide opportunities to identify poor practice
systematically and also to provide opportunities for learning and sharing, and
as such, form an element of MI staff’s continuing professional development. In
addition, the aim is that all MI centres are externally audited against
relevant national standards every 3 years. An audit tool has been developed to
document the standards that centres have achieved, to identify improvement over
time and also to provide a pathway for achieving excellence.
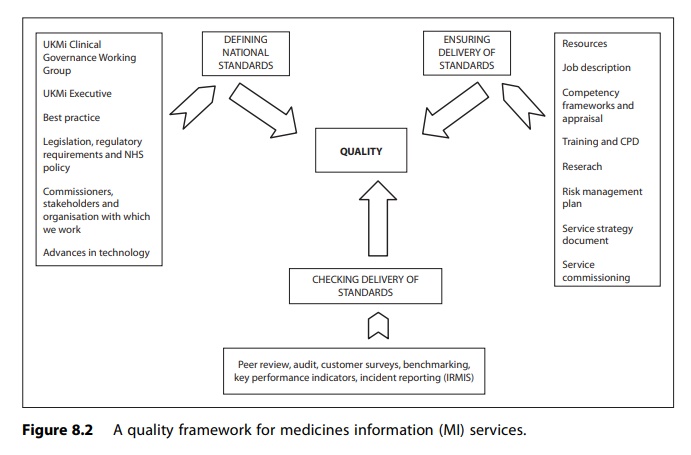
The framework
developed in the UK for achieving quality within MI services through
implementing an appropriate clinical governance process is outlined in Figure
8.2.
In addition to
improving their own performance, MI pharmacists are well placed to contribute
to a wider clinical governance agenda within the NHS by supporting
evidence-based practice across pharmacy and the wider trust, for example
supporting formularies and contributing to educational programmes and knowledge
management systems.
Customers and users
The historical user
base of MI services has been principally drawn from hospital healthcare
professions, mainly from pharmacy, medicine and nursing. Clinical pharmacists
are often the largest user group of a local MI service because ward-based
clinical pharmacists have a high profile in prob-lem-solving and facilitating
optimum patient care. Therefore, they act as the primary contact for
medicines-related issues, for which MI services provide their first-line
back-up. However, there are many situations in which a clinical pharmacist is
not available for dealing with such issues, such as in less acute clinical
areas and outpatient clinics, when direct contact by the doctor or nurse with
the MI service is appropriate. More recently, independent non-medical
prescribers – nurses and pharmacists – have emerged to take over some
traditional medical prescribing. MI services have a significant role in
training and supporting the prescribing activities of these new groups. There
are also many other hospital-based healthcare professions that have an
interaction with drug therapy and who therefore may need to utilise the MI
service; such groups include dieticians, physiotherapists, psychologists and
laboratory services.
MI services have
expanded their activities to include primary care. The majority of this
activity is directed towards those groups with direct patient care
responsibilities, general practitioners, community pharmacists, nurses
(community, practice, midwives) and health visitors. However, profes-sional
advisers in primary care organisations, such as primary care trusts, also
require MI services support to facilitate safe and cost-effective pre-scribing.
This more strategic role is largely being provided from regional MI services.
It is important that MI services recognise the critical nature of the link
between primary and secondary care, thereby facilitating the transparent and
seamless treatment of patients, wherever they receive their care.
Patients have a high
requirement for information on medicines and this is provided in a number of
ways. All patients, whether in hospitals or in the community, should receive a
patient information leaflet with any licensed, dispensed medicines. This, in
many circumstances, is augmented with counselling from a hospital or community
pharmacist. NHS Direct (England and Wales) and NHS24 (Scotland) are telephone
and internet services established to provide the general public with instant
information on any aspect of healthcare, including medicines. Although these
services have trained staff and limited resources to deal with
medicines-related
issues, they utilise
MI services to deal with more complex clinical issues. In addition, in the UK,
local MI services are providing medicines/patients telephone helplines for
patients who have received medi-cines, either as inpatients or outpatients, so
that specific enquiries regarding their hospital-dispensed medication, or more
general issues, can be resolved. These services therefore are aimed at
improving both safety and concordance.
Other groups, such
as the police, coroners, self-help groups and the media, also have a
requirement to use MI services, although there are specific guide-lines for
dealing with such groups which address issues such as confidentiality.
Different professional groups, and levels and specialties within these groups,
have differing requirements of the service, both in term of reactive clinical
problem-solving and educational and current awareness services, and this is an
important feature for MI services to recognise.
Related Topics
