Configuration of Chiral Centers
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Stereochemical and Conformational Isomerism
As noted above, when a single chiral center is identified in a molecule, then there will be two stereoisomers (enantiomers) of that molecule. Enantiomers differ only by the spatial arrangements of groups around the chiral center; however, they are distinct isomers.
CONFIGURATION OF CHIRAL CENTERS
As
noted above, when a single chiral center is identified in a molecule, then
there will be two stereoisomers (enantiomers) of that molecule. Enantiomers
differ only by the spatial arrangements of groups around the chiral center;
however, they are distinct isomers. To discuss enantiomers, it is necessary to
have some way to name or denote which one is which; that is, we have to convert
the configuration (spatial arrangement) of groups around the chiral center into
a name or designation. An early method was to designate enantiomers as either D
or L based on the relationship of their chiral center to the chiral center in
D- or L-glyceraldehyde. This system of nomenclature was difficult to apply to
larger molecules and particularly ones with more than one chiral center.
In
response to this nomenclature dilemma, the Cahn – Ingold – Prelog (IUPAC,
International Union of Pure and Applied Chemistry) system of nomenclature was
developed and is now the standard method to specify the relative configuration
of chiral centers in molecules. Each chiral center will have two possible
mirror-image configurations, which are designated as either R or S.
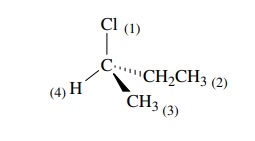
The
strategy for determining whether the chiral center has an R or S config-uration
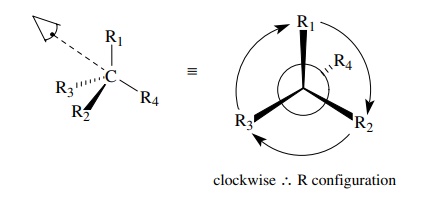
is based on the symmetry properties of a tetrahedral carbon. First one assigns
priorities to the four groups attached to the chiral center. Next one orients
the molecule so as to sight directly down the bond from the chiral carbon to
the group of lowest priority. The remaining three bonds will form a trigonal
array (as in Newman projections).
(If
it is not apparent from stereostructures, build a model and satisfy yourself
that this is so.)
If
you then follow these three groups around a circle from the group of highest
priority to the group of lowest priority, you must proceed either clockwise
(right), which is designated as the R configuration, or counterclockwise
(left), which is designated as the S configuration. This provides a general
method for assigning the configuration to any chiral center.
In
summary:
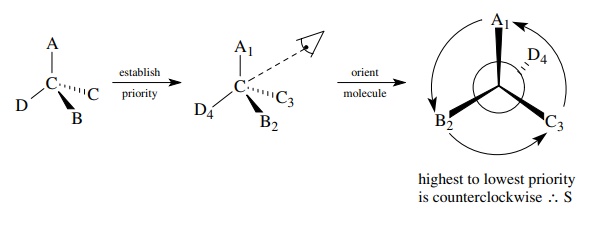
1. Assign priorities to the groups attached to the chiral
center.
2. Orient the molecule so the group of lowest priority
points directly away from your eye.
3.
Follow the direction of the remaining groups from the highest to lowest
priority. If the procession is clockwise, the configuration is designated R; if
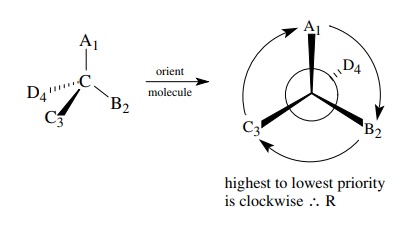
the procession is counterclockwise, the configuration is designated S.
The
other enantiomer will obviously have the R configuration.
The
only remaining task is to assign priorities to the groups attached to the
chiral center. This is done by the following rules:
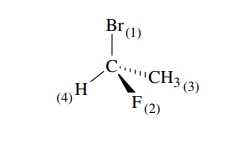
1.
Priority is first assigned on the basis of the atomic number of the atoms
attached directly to the chiral center. Atoms of higher atomic number are given
higher priorities. Thus for 1-bromo-1-fluoroethane, the ordering of priorities
is Br > F > C > H on the
basis of their respective atomic num-bers of 35 > 9 > 6 > 1.
2.
When assignment of priority cannot be made on the basis of atoms attached
directly to the chiral center, proceed away from the chiral center and exam-ine
the next sets of atoms for differences in atomic numbers of attached atoms.
Thus for 2-chlorobutane, two carbon atoms are attached to the chi-ral center.
To establish which carbon group takes priority, note that the next atoms are
H,H,H for the methyl group and H,H,C for the ethyl group. Thus the latter has
higher priority because of the greater atomic number of carbon.
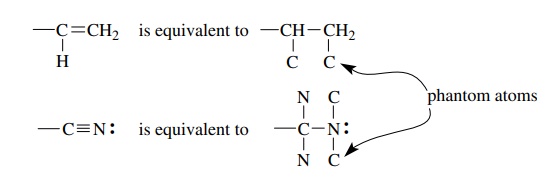
3. Groups containing multiple bonds are assigned priority as if both atoms were doubled or tripled. Thus a vinyl group is equivalent to a 2-butyl group by so-called phantom atoms. The phantom atoms do not include the requisite number of hydrogen atoms to complete the valences.
These
rules and applications are summarized completely in most introductory organic
texts. It is important to be able to assign R,S configurations to stereogenic
centers in molecules and to construct chiral molecules given the R or S
config-uration. In this way it is very easy to determine the stereochemical
relationships between stereoisomers.
Related Topics
