The coinage metals
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : Transition Metals and d-Block Meta Chemistry
General chemistry 1. Copper-containing drugs 2. Silver: the future of antimicrobial agents? 3. Gold: the fight against rheumatoid arthritis - Essentials of Inorganic Chemistry : Transition Metals and d-Block Meta Chemistry
The
coinage metals
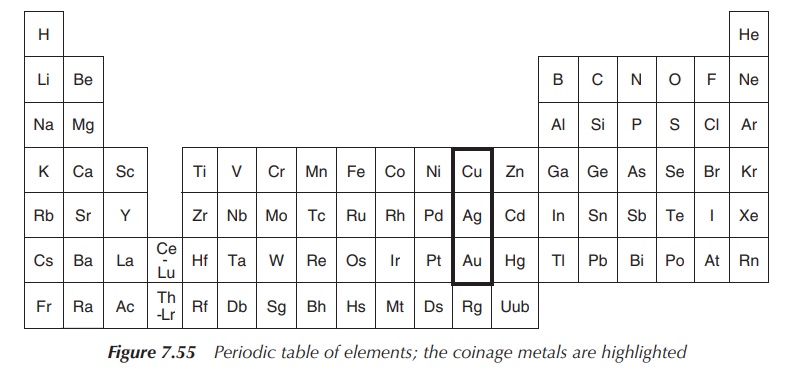
Historically, the three nonradioactive members of group 11 of
the periodic table (11th vertical column) are designated as coinage metals,
consisting of copper (Cu), silver (Ag) and gold (Au) (Figure 7.55).
Metals used to make coins have to fulfil special requirements,
considering that a coin may be in circulation for several decades. Therefore, a
coin needs to have anticorrosive properties and should not show any
signifi-cant signs of wear. Very often, coinage metals are therefore mixed with
other metals to form a so-called alloy. These alloys are harder and often more
resistant to everyday use than the metals themselves.
Quite often, there is a problem that for low denomination coins
the face value is significantly lower than the value of the metal itself. For
example, the modern British penny is made out of steel plated with copper,
whereas the American penny is made out of zinc with a copper covering.
General chemistry
Group 11 metals, such as Cu, Ag and Au, are known as the noble metals and belong to the d-block
or transition metals. They are all relatively inert and corrosion-resistant
metals and therefore are useful for the production of coins. All three metals
are excellent conductors of electricity and heat. The most conductive metal for
electricity is Ag, followed by Cu and then Au. Silver is the most thermally
conductive element and the most light-reflecting element. Copper is widely used
in electrical wiring and circuitry. In precision equipment, where the risk of
corrosion needs to be kept as low as possible, gold is quite often used. Silver
is widely used in mission-critical applications such as electrical contacts as
well as in agriculture, medicine and scientific applications. Probably, one of
the best known applications of silver ions and silver is the use in
photography. Upon exposure, the silver nitrate in the film reverts to metallic
silver itself.
Silver, gold and copper are all quite soft metals and therefore
are not very useful as weapons or tools.
Nevertheless, because of their malleability, they have been and
still are used to make ornaments and jewellery.
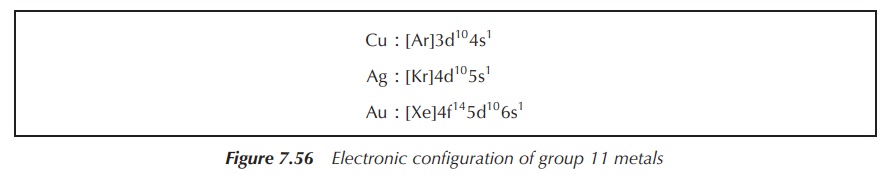
The electronic configuration of group 11
metals is shown in Figure 7.56.
Silver and gold are generally inert and not attacked by oxygen
or nonoxidising acids. Silver dissolves in salpetric acid, and in the presence
of H2S it forms Ag2S. Gold dissolves in concentrated
hydrochloric acid, forming [AuCl4]− in the presence of an
oxidising agent.
Both metals react with halogens. Ag(I), Au(III) and Au(I) are
the dominant oxidation states, whereas the dominant oxidation states of copper
are Cu(II) and Cu(I). Typical examples for the oxidation states III include AuF3,
AuCl3 and AuBr3, whereas there is only AgF3.
There are many examples for silver and gold salts where the metal takes the
oxidation state I.
Copper-containing drugs
Copper is a valuable metal and has been mined for more than 2000
years. It has had many uses throughout history. Initially, copper was mainly
used to make alloys such as brass and bronze, which are harder and stronger
than copper itself. Nowadays, copper is mainly used because it conducts heat
and electricity (e.g. wiring) and it is corrosion-resistant (e.g. as roofing
material).
Historically, copper was used for the treatment of a variety of
diseases, including chronic ulcers, headaches, ear infections, rheumatoid
arthritis (RA), and so on. In 1832, copper workers were found to be immune to
an outbreak of cholera in Paris, which stimulated further research into the
medicinal use of copper. Almost every cell in the human body uses copper, as
most contain copper-dependent enzymes. Unfortunately, excessive amounts of
copper are toxic for the human body, whereas low amounts of copper also lead to
health problems, manifested in Menkes disease.
Copper ions from food sources are processed by the liver, and
transported and excreted in a safe manner. Inorganic metallic copper from
sources such as drinking water mainly enters the blood directly and can be
toxic as it can penetrate the blood–brain barrier. Typically, 50% of the daily
copper intake is absorbed in the GI tract and transported to the liver from
where it is transported to the peripheral tissue bound to ceruloplasmin, a
copper-binding glycoprotein. A smaller amount of copper is also bound to
albumin. Excess copper is mainly excreted in bile into the gut and then the
faeces.
Copper is an essential trace metal, and copper
ions are incorporated into a number of metalloenzymes – so called cuproenzymes. In the human body, the
majority of copper ions can be found as Cu2+; nevertheless, the
oxidation state shifts between the cuprous (Cu+) and cupric (Cu2+)
forms. It is important that copper can accept and donate electrons easily for
its role in a variety of cuproenzymes, and examples are outlined in the
following. Lysyl oxidase, a cuproenzyme, is responsible for the cross-linking
of collagen and elastin, which forms strong and at the same time elastic tissue
connections. These tissues are used, for example, for the formation of blood
vessels and the heart. Ceruloplasmin (ferroxidase I) and ferroxidase II, two
copper-based enzymes, can oxidise ferrous iron to ferric iron. Ferric iron can
then be transported with the help of trans-ferrin, for example, to form red
blood cells. Furthermore, a variety of copper-dependent enzymes, such as
cytochrome c and superoxide dismutase, work as antioxidants and are involved in
the reduction of reac-tive oxygen species (ROS). There are also a variety of
cuproenzymes that are involved in the synthesis (dopamine-b-monooxygenase) and
metabolism (monoamine oxidase) of neurotransmitters .
1. Wilson disease
Wilson disease is a genetic disorder in which excessive amounts
of copper build up in the human body. The copper is mainly stored in the liver
and brain, and therefore causes liver cirrhosis and damage to the brain tissue.
The damage to the brain tissue occurs mainly at the lenticular nucleus and a
typical brown ring is visible around the iris; therefore Wilson disease is also
called hepatolenticular degeneration.
Wilson disease was first described in 1912 by Wilson, pointing
out the ‘progressive lenticular degeneration’ accompanied by chronic liver
disease, which was leading to cirrhosis. It was Kayser and Fleischer who later
made the association between these symptoms and a deposition of copper in the
human body. Later on, it was established that Wilson disease is a genetic
disorder with an autosomal recessive inheritance pattern. The abnormal gene was
identified to be ATP7B, a metal-transporting adenosine triphosphatase (ATPase)
mainly expressed in hepatocytes, which has the main function of transporting
copper across the membrane. This means that, in the absence of this gene, the
excretion of copper via the liver is reduced. This leads to copper accumulation
in the liver, which damages the liver and eventually releases copper into the
blood stream from where it can further poison the organs. Mainly the brain,
kidneys and the cornea are affected by the copper accumulation.
Wilson disease presents itself mostly as a liver disease or a
psychological illness and was fatal until treatments were developed. The main
route of treatment is chelation therapy (see Chapter 11), with British
anti-lewisite (BAL) being the first chelating agent used in 1951. In 1956, the
orally available chelating agent d-penicillamine simplified the treatment of
Wilson disease. Further treatment options include liver transplant, which has
the potential to cure these patients . Zinc supplementation can also be used in
patients with Wilson’s disease, as it prevents the absorption of copper ions.
It is important to note that this therapy has a slow onset time and chelating
treatments have to be continued for 2–3 weeks after the start with zinc
supplementation .
2. Copper and wound healing
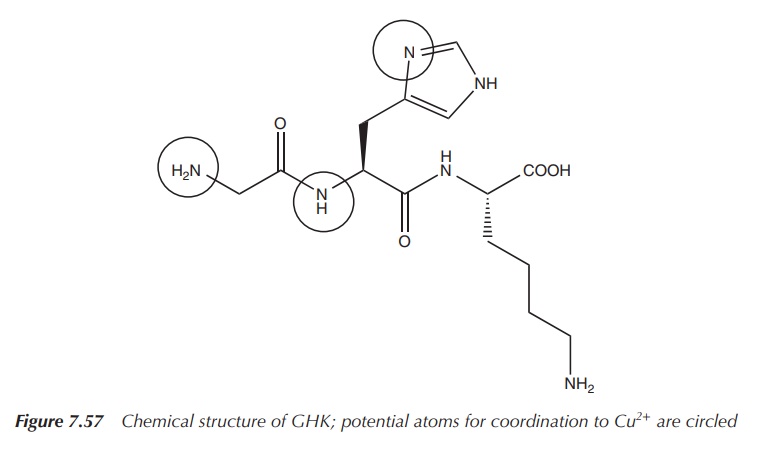
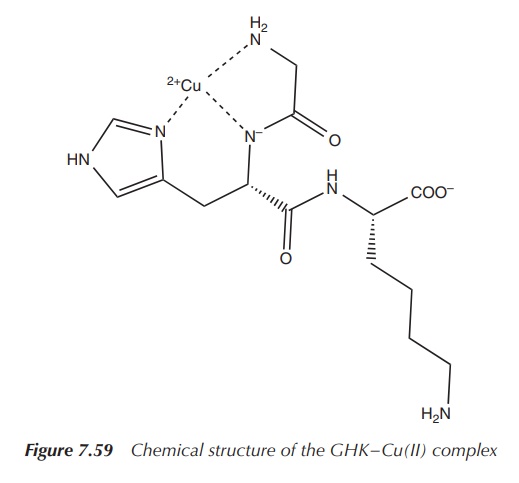
Glycyl-l-histidyl-l-lysine (GHK) is a tripeptide known for its
high binding affinity to Cu2+ and its complex role in wound healing.
The GHK–Cu(II) complex was isolated from human plasma in the 1970s and it was
shown to be an activator for wound healing. GHK–Cu(II) has two main functions:
as an anti-inflammatory agent to protect the tissue from oxidative damage after
the injury, and as an activator for wound healing itself as it activates the
tissue remodelling .
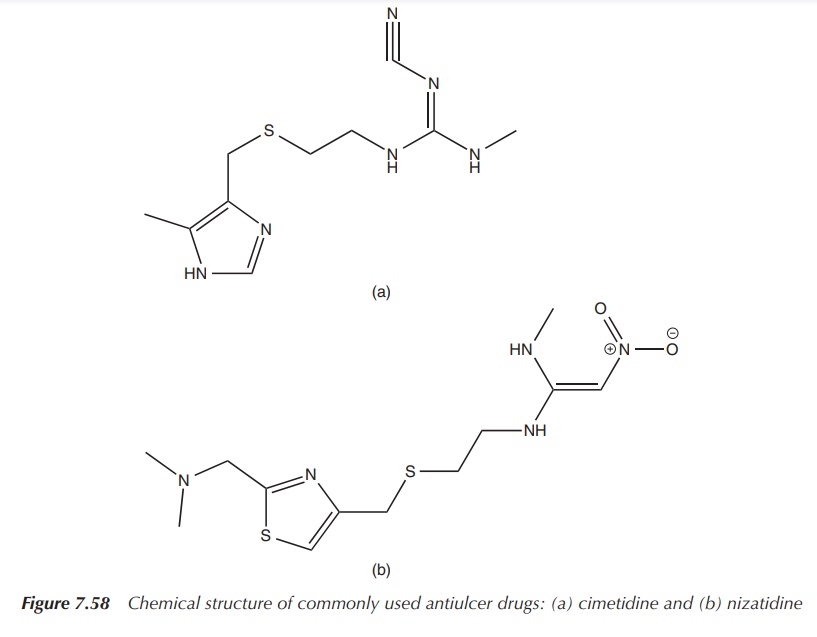
The structure of GHK is very similar to that of common drugs
used to treat ulcers (Figures 7.57–7.59). After the initial stages of wound
healing are activated, such as blood coagulation and neutrophil invasion, a
second stage of wound healing begins, which includes the population of GHK at
the wound, which has a high affinity to Cu2+. Mast cells, which are
located in the skin, secrete GHK, which accumulates Cu2+ and forms
the copper complex GHK–Cu(II) and therefore increases the metal–tripeptide
concentration at the wound. First, GHK–Cu(II) has an anti-inflammatory effect
by protecting the tissue from oxidative damage and by suppressing local
inflammatory signals (i.e. cytokine interleukin-1 (IL-1)). Second, GHK–Cu(II) is
released into the blood stream and encourages the production of wound
macrophages that support the wound repair by removing the damaged tissue and
secreting a family of several growth factor proteins. GHK–Cu(II) also hinders
fibroblast production of TGF-β-1 and therefore suppresses the scar development.
The GHK–Cu(II) complex also stimulates the growth of blood vessels, neurons and
elastin, and, in general, supports most processes of wound healing .
Because of its versatile properties during the
wound-healing process, it is not surprising that researchers tried to design
commercial products based on GHK–Cu(II). Initial results were promising, but
unfortunately the stability of the tripeptide GHK was not sufficient enough
resulting in rapid breakdown. In the human body, GHK is permanently reproduced
and therefore stability issues are not a major problem .
3. Copper and cancer
Cancer progression has been linked to increased ceruloplasmin
and copper levels in a variety of tissues. Copper deficiency has been
considered as an anticancer strategy, but several clinical studies have not
been encouraging. The exact role of copper in cancer is not yet fully
understood, but it is possible to be involved via oxidation processes and the
production of ROS and its involvement in angiogenic processes, as copper is
believed to stimulate proliferation of endothelial cells .
Silver: the future of antimicrobial agents?
The name silver is derived from the Saxon word ‘siloflur’, which
has been subsequently transformed into the German word ‘Silabar’ followed by
‘Silber’ and the English word ‘silver’. Romans called the element ‘argentum’,
and this is where the symbol Ag derives from.
Silver is widely distributed in nature. It can be found in its
native form and in various ores such as argentite (Ag2S), which is
the most important ore mineral for silver, and horn silver (AgCl). The
principal sources of silver are copper, copper–nickel, gold, lead and lead–zinc
ores, which can be mainly found in Peru, Mexico, China and Australia.
Silver has no known active biological role in the human body,
and the levels of Ag+ within the body are below detection limits.
The metal has been used for thousands of years mainly as ornamental metal or
for coins.
Furthermore, silver has been used for
medicinal purposes since 1000 BC. It was known that water would keep fresh if
it was kept in a silver pitcher; for example, Alexander the Great (356–323 BC)
used to transport his water supplies in silver pitchers during the Persian War.
A piece of silver was also used, for example, to keep milk fresh, before any
household refrigeration was developed. In 1869, Ravelin proved that silver in
low doses acts as an antimicrobial. Around the same time, the Swiss botanist
von Nägeli showed that already at very low concentration Ag+ can
kill the green algae spirogyra in fresh water. This work inspired the
gynaecologist Crede to recommended use of AgNO3 drops on new born
children with conjunctivitis.
In 1884, Crede introduced the application of a 1% silver nitrate
solution for the prevention of blindness in newborn, and the results were so
impressive that this still used nowadays in America
Today, airlines and NASA rely on silver filters to guarantee
good water quality on board their aircrafts. A contact time of hours is
necessary to see a disinfectant against coliforms and viruses of silver in
water. In the concentrations normally applied, silver does not show any impact
on the taste, odour or colour of the water. There is also no negative effect on
human cells known. The only negative health effect known is called argyria, which is an irreversible
darkening of the skin as a result of a prolonged application of silver. The existence of silver-resistant organisms
is probably one of the major drawbacks for silver-based therapies.
1. Silver ions and their medicinal use
The antibacterial activity of silver and silver ions has long
been known and led to many applications. This is mainly due to the fact that
its toxicity to human cells is considerably lower than to bacteria. Most
commonly, it is used for the prophylactic treatment of burns and in water
disinfection. Unfortunately, the mechanisms and the chemistry of silver ions in
biological organisms are so far not entirely clear. von Nägeli proposed the
so-called oligodynamic effect, which describes the toxic effect of metals on
organisms. It has been proposed that silver ions irreversibly damage key
enzymes in the cell membrane of bacteria. This would lead to an inactivation of
the pathogen. Silver reacts, as many other transition metals, preferentially
with the thiol groups and also amino, carboxyl, phosphate and imidazole groups.
Silver nitrate (AgNO3), after salicylic acid, is
widely used for the treatment of warts. AgNO3 is a highly water-soluble
salt, which readily precipitates as AgCl, black in colour, when in contact with
the skin. Warts are caused by a human papillomavirus, and mostly hands, feet
and the anogenital areas are affected. The treatment is based on the
destruction of the local tissue, and the silver salt is applied via a caustic
pen to the affected area. Silver nitrate is highly corrosive and is known to
destroy these types of tissue growth. Care has to be taken when this treatment
option is used, as the resulting AgCl stains any skin or fabric which it has
been in contact with.
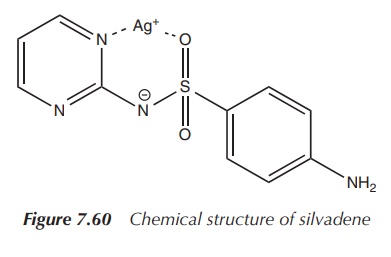
2. Silver(I) sulfadiazine
Silver sulfadiazine is indicated for the prophylaxis and
treatment of infections in burn wounds. Silver sulfa-diazine is highly
insoluble in water, and as a result, it does not cause hypochloraemia in burns
in contrast to silver nitrate.
The active ingredient silver sulfadiazine is a sulfur-derived
topical antibacterial used primarily on second-and third-degree burns. It is
known to be active against many Gram-negative and Gram-positive bacteria as
well as against yeast. The cream is kept applied to the burned skin at all
times for the duration of the healing period or until a graft is applied. It
prevents the growth of a wide array of bacteria, as well as yeast, on the damaged
skin. Caution has to be given to large-area application as sulfadiazine levels
in the plasma may well reach therapeutic levels that can cause side effects
(Figure 7.60).
3. Silver dressings
All kinds of dressings containing silver ions have become more
and more popular because of their antimicro-bial effect. Nevertheless, the
effect of silver nanoparticles on wound healing is still under discussion.
Also, the use of dressings containing silver sulfadiazine and hydrocolloid
dressings containing silver for the treatment of foot ulcers is still under
discussion .
So far, clinical recommendation suggests that antimicrobial dressings containing silver should be used only when there are clinical signs of an infection present. They should not be routinely used for wound dressing or the treatment of ulcers.
Silver dressing will work only in
the presence of wound secretion, as only then the silver ions will show an
antimicrobial effect. There is also some evidence that silver dressings delay
the healing process of acute wounds and therefore silver dressings are not
recommended for use in those cases .
Gold: the fight against rheumatoid arthritis
From ancient times, gold has been regarded as one of the most
beautiful metals and ever since treasured by man. The metal was first used to
make tools, weapons and jewellery but was soon used for trade and as coins.
Gold is a soft yellow metal, which is characterised by its high ductility. Very
often, gold is alloyed with other metals to give it more strength. For example,
white gold is gold alloyed with palladium.
Gold has also a long-standing tradition in medicine, as it has
been used by many nations for thousands of years. From as early as 2500 BC,
Arabians, Chinese and Indians used gold compounds for medicinal purposes. In
mediaeval times, the elixir ‘aurum potabile’, which was an alcoholic mixture of
herbs with some gold flakes, was sold by medicine men travelling around Europe
and this elixir was supposed to cure most diseases. In the nineteenth century,
Na[AuCl4] was reported to treat syphilis, whilst others used it to
cure alcoholism. On a more serious note, Koch discovered in 1890 the
antibacterial properties of gold cyanide. In
vitro experiments with the Mycobacterium
tuberculosis showed that gold cyanide has the potential as a tuberculosis
therapy. Gold compounds were also investigated for the treatment of RA, when it
was believed that RA was caused by bacteria, and many other health problems .
1. Gold therapy for rheumatoid arthritis
RA is a chronic, inflammatory, progressive autoimmune disease
that primarily affects the joints. The disease is characterised by swelling of
the joints and increasing pain leading to stiffness. Inflammation occurs
initially in the synovial membrane surrounding the joints and then spreads to
the synovium. An irreversible erosion of the articular cartilage on the bone
joints means that bones will directly rub against each other and cause severe
pain. The peak period of onset is between 35 and 55 years of age, with
premenopausal women more often affected than men (ratio of 3 : 1). There is no
obvious inheritance pattern to be found, but a genetic predis-position seems to
be underlying. Patients are diagnosed on mainly three factors: painful joints,
inflammation and the presence of the so-called rheumatoid factor.
Treatment options include anti-inflammatory drugs and/or
disease-modifying antirheumatic drugs (DMARDs). Anti-inflammatory drugs
comprise mostly nonsteroidal anti-inflammatory drugs (NSAIDs) or corticosteroids.
NSAIDs are the first drugs of choice for the treatment of mild RA, as they
possess analgesic and anti-inflammatory properties and also can be given in
conjunction with DMARDs. Patients respond and tolerate NSAIDs quite variably,
but generally the onset is rather quick. If there is no relief within 2–3
weeks, the treatment options are reconsidered. Corticosteroids are the most
potent anti-inflammatory agents and additionally can be used as
immunosuppressants. Corticosteroids are very commonly used in RA despite their
severe side effects, which really limit their application. Additionally,
corticosteroids have very little influence on the disease itself, but they are
invaluable when the pain gets intolerable and mobility is severely decreased.
DMARDs are a category of unrelated drugs that
are used in RA to slow down the progression of the disease. In contrast, NSAIDs
treat only the inflammation, and corticosteroids are also insufficient to slow
down the progression of RA. DMARDs are comprised of immune modulators,
sulfasalazine and gold compounds amongst others, and they are used when the RA
progresses from a mild to a more severe form. The onset time for DMARDs is
significantly longer than for NSAIDs and it can take 2–6 months until any
effect is seen. Typically, irreversible joint damage is already observed in the
2 years following diagnosis of RA, and therefore it is recommended to consider
DMARDs as soon as moderate to severe RA is diagnosed. Some patients respond
well to a combination therapy of NSAIDs and DMARDs, but patient response varies
from individual to individual. In general, DMARDs have a higher toxicity and
therefore come with significantly more side effects than NSAIDs. Gold salts are
long known for their therapeutic effects in RA. In general, the application of
gold compounds to treat diseases is called chrysotherapy,
with RA being the main application area. Gold salts are clinically available as
oral and intramuscular formulations.
Chrysotherapy is the treatment of certain diseases,
especially RA, with gold compounds. Side effects can be quite severe and dose-limiting and include discolouration of
skin, diarrhoea, nausea, flushing, vomiting, metallic taste in mouth and even
damage of the kidneys and liver .
2. Examples of gold-containing DMARDs
Au(I)thiolates, such as aurothioglucose,
disodium aurothiomalate and trisodium bis(thiosulfato)gold, are the
first-generation gold-based DMARDs. They feature linear, two-coordinated Au(I)
thiolates and are polymeric with the exception of trisodium bis(thiosulfato)gold.
The thiolate group stabilises the oxidation state of +1 for the gold atom, and
therefore hinders disproportionation to Au(O) and Au(II).
Disproportionation is a redox reaction in which a species is
oxidised and reduced at the same time and
two different products are formed.
The first-generation gold drugs are water soluble because of
their hydrophilic groups and they are therefore commonly administered by
intramuscular injection. The injection has to be done by a healthcare
professional, which means the patient requires regular visits to the clinic.
These gold-based compounds typically accumu-late in the kidneys, where they are
nephrotoxic and cause a leakage of proteins at the glomerulus. Typically,
chrysotherapy is discontinued if there is no beneficial effect seen after 6
months, but side effects often last longer.
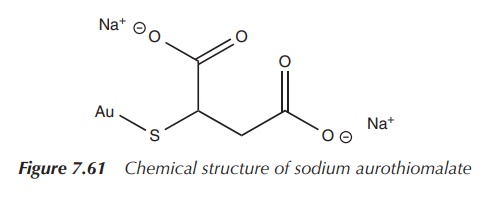
Sodium aurothiomalate is a commonly used gold-based DMARD and is indicated for active progressive RA. It is administered by deep intramuscular injection. Administration is started with a test dose of 10 mg followed by weekly intervals of 50 mg doses. An improvement is expected to be seen once 300–500 mg is administered. Treatment should be discontinued if there is no improvement after administering 1 g or 2 months. Intervals of administration should be gradually increased to 4 weeks in patients in whom an effect can be seen. If any blood disorders or other side effects such as GI bleedings or proteinuria are observed, sodium aurothiomalate should be discontinued [8, 27] (Figure 7.61).
A second-generation gold-based drug came on the
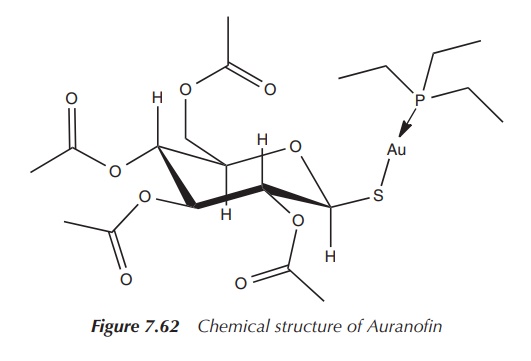
market, called Auranofin – ([tetra-O-acetyl- β-D-(glucopyranosyl)thio]-triethylphosphine)gold(I)
– licensed as an orally available gold drug for the treatment of RA. It features
a linear S–Au–P geometry, as shown by X-ray analysis. It is more lipophilic
than the first-generation drugs, which makes oral administration possible.
Treatment with Auranofin requires less visits to the clinic, but it is believed
to be less successful in the treatment of RA compared to gold drugs being
administered intramuscularly (Figure 7.62).
3. Metabolism of gold drugs
The clinically used gold drugs can be considered as prodrugs,
which undergo rapid metabolism in vivo
to form active pharmacological species. The precise mechanism of action is not
known, but most probably it involves a thiol exchange. This means that the
thiolate ligand will be replaced by a biological thiol such as albumin, which
is a major protein in the serum and is sulfur-rich. Cysteine-34, one of the
cysteine residues in albumin, is likely to be deprotonated at physiological pH
and is a likely target for the gold compounds. In the case of Auranofin, the
triethylphosphine (Et3P) is oxidised, whilst the disulfide link in
the albumin is reduced, and the harmless oxidised species Et3P==O is excreted via the kidneys.
The water-soluble first-generation gold drugs are injected intramuscularly and interact with the cells directly. They do not enter the cells but bind to the cell membranes via thiol groups on the cell surface and interfere with normal cell signalling pathways. Orally available gold drugs, such as Auranofin, enter the cells by a so-called thiol shuttle. Auranofin is transported to the cell, where it reacts with sulfhydryl-dependent membrane transport proteins (MSH), which are present on the cell membrane.
The Et3PAu+ moiety binds to MSH and is
transported into the cell, where the phosphine is oxidised as explained earlier
and the Au moiety binds to the proteins. The thiolate ligand remains outside
the cell. The gold cation can also leave the cell using the same mechanism,
independent of the phosphine ligand.
4. Further pharmacological potential of gold complexes
The major clinical use of gold complexes
relates to RA. Nevertheless, screening for the antitumour activity of antiarthritic gold drugs has encouraged
studies towards the use as anticancer drugs. Au(III) species are isoelectronic
to cisplatin (the most widely used metal-based anticancer drug).
The
term isoelectronic describes two or
more molecular entities that have the same number of valence electrons and the
same chemical structure independent of the actual elements present.
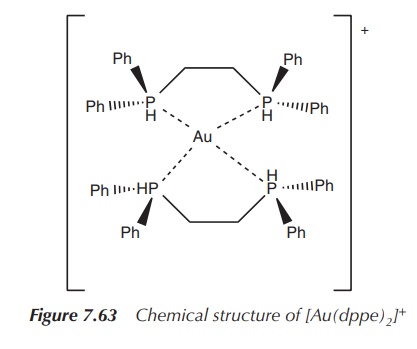
The Au(I) complex of bis(diphenylphosphino)ethane (dppe) has an
exciting cytotoxic profile when tested in
vitro and in vivo. Experiments
have shown that it was more effective when co-administered with cisplatin than each of the compounds
individually. Unfortunately, no further studies were undertaken; especially,
clin-ical trials could not proceed, as an acute toxicity to heart, liver and
lungs was detected. Furthermore, some antimicrobial
activity and some antimalarial activity
of Au(I) complexes have been reported
(Figure 7.63).