Electron Movement
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Curved-Arrow Notation
In a very simple sense, most organic reactions are accomplished merely by the movements of electrons.
ELECTRON MOVEMENT
In
a very simple sense, most organic reactions are accomplished merely by the
movements of electrons. Since molecules are composed of atoms held together by
bonds and covalent bonds are merely shared pairs of electrons, the conversion
of one molecule to another by changes in chemical bonds between reactants and
products can be described simply as changes in electron pairs that are shared
between the various nuclei in the reactants. While this is a gross
oversimplifica-tion, it nevertheless provides us with a very important tool
with which to keep track of bonding changes that occur during the
transformation of one molecule into another.
We
keep track of electron pairs by noting changes in their location in molecules
by means of curved arrows. The curved arrow depicts movement of an electron
pair from the tail of the arrow to the head of the arrow. For example,
in the reaction of a proton with water to produce the hydronium ion, a new bond
is formed from oxygen to hydrogen. The electrons in that bond start out as a lone
pair on oxygen and are donated to and shared with the proton. We can easily
show this electron movement with a curved arrow from the lone pair on oxygen to
the proton.
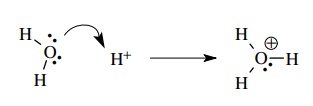
In
a similar sense the Lewis acid – base reaction between ammonia and boron
trifluoride can be depicted by a curved arrow from the lone pair of electrons
on nitrogen to the boron atom. This electron movement creates a new bond
between nitrogen and boron, but the curved-arrow notation clearly illustrates
that the electron pair of that bond is supplied by the nitrogen. An additional
consequence is that the nitrogen atom gains a formal positive charge and the
boron atom gains a formal negative charge.
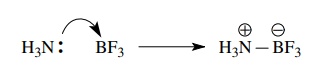
Before
further applications of electron movement using curved-arrow notation are
presented, it is important to recognize just why it is such a useful tool for
describing reactions. First it permits us to keep track of valence shell
electrons during a chemical reaction and thus serves as a method for electronic
bookkeep-ing. Second it shows how changes
in bonding result from changes in electron distribution. Third it can show
likely mechanisms for chemical reactions in terms of the breaking and making of
chemical bonds.
For
curved-arrow notation to be used correctly, however, the structural and bonding
principles which we have already learned must be adhered to. Thus donor –
acceptor properties, oxidation states, hybridization and the octet structure of
atoms, normal valences, formal charges, reactive intermediates, and so on, must
all be taken into account as curved-arrow notation is used to track changes in
electron distribution that occur during chemical reactions. Within the
frame-work of these principles, however, curved-arrow notation can be a
powerful and effective tool for depicting bonding changes during chemical
reactions. To do this, it is necessary to first understand the general
processes of bond formation and bond cleavage that are commonly encountered.
Related Topics
