Electronic Effects
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Mechanisms of Organic Reactions
Besides bond breaking, another common feature of many reactions is the formation of charged species as intermediates. Carbocations, carbanions, oxonium ions, and so on, are all commonly encountered intermediates formed in the rate-determining step of multistep reactions.
ELECTRONIC EFFECTS
Besides
bond breaking, another common feature of many reactions is the formation of
charged species as intermediates. Carbocations, carbanions, oxonium ions, and
so on, are all commonly encountered intermediates formed in the
rate-determining step of multistep reactions. As a consequence, charge
development in the activated complex is expected. In terms of the reaction
mechanism, it is very important to know the charge type (positive, negative, or
none) and the extent of charge development in the activated complex.
The
use of rate constants can provide a clue to charge development as well. Changes
in the rate constant of a reaction due to changes in structure can be
indicative of the charge distributions present in the activated complex. For
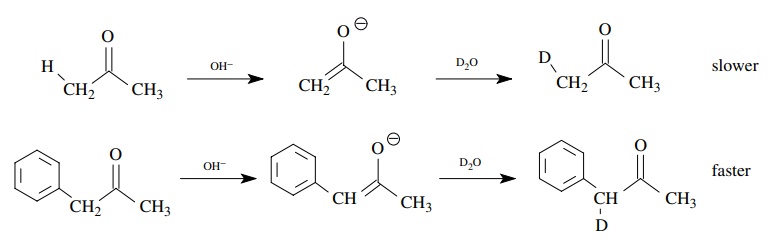
example, rate constants are much larger for the base-promoted deuterium
exchange of phenylacetone than for acetone itself because the phenyl group
stabilizes the negative charge on the enolate ion (and the transition state
leading to it). Hence the α proton is
removed more rapidly and deuterium exchange is speeded up correspondingly. This
behavior is entirely consistent with an increase of electron density on the α carbon during the rate-determining
step.
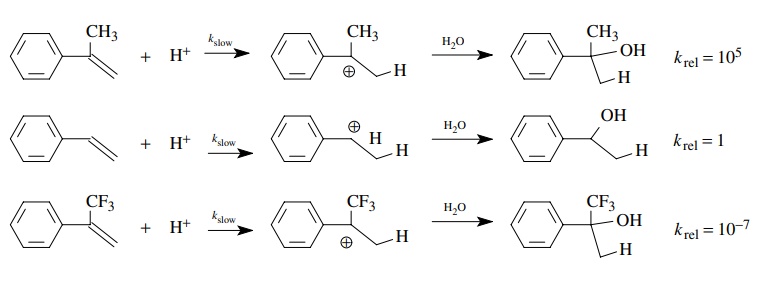
The
hydration of styrene, α-methylstyrene,
and α-trifluoromethylstyrene gives a
benzylic alcohol product; however, α-methylstyrene
reacts 105 more rapidly than styrene itself, while α-trifluoromethylstyrene reacts 107
less rapidly than styrene. This behavior is consistent with the rate-determining
step being pro-tonation of the double bond to give a carbocation. The
developing positive charge in the transition state is stabilized by the
inductive effect of the methyl group in α-methylstyrene,
the transition state is of lower energy, and it thus reacts faster. The
developing positive charge in the transition state is destabi-lized by the
electron-withdrawing inductive effect of the trifluoromethyl group in α-trifluoromethylstyrene, the transition
state is of higher energy, and thus it reacts more slowly.
While
changes in rate constants in response to changes in structure are extremely
valuable for indicating the type of charge development occurring in the
activated complex, the actual extent of charge development in the activated
complex is an additional structural descriptor that would be very useful.
Related Topics
