Electrophilic Carbon
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Carbon-Carbon Bond Formation Between Carbon Nucleophiles and Carbon Electrophiles
Carbon-centered electrophiles are compounds or intermediates which are electron poor and thus capable of accepting electrons from electron donors.
ELECTROPHILIC CARBON
Carbon-centered
electrophiles are compounds or intermediates which are electron poor and thus
capable of accepting electrons from electron donors. To be an electron
acceptor, an electrophile must have an unfilled orbital on carbon avail-able
for overlap with a filled orbital of the donor. Unfilled atomic p orbitals or
antibonding orbitals (both σ ∗ and π ∗ ) are the most common types of acceptor orbitals. The most
common carbon electrophiles fall into four major categories:
(a) Cationic carbon electrophiles are the most reactive
because of the positive charge they carry. They can, however, have a variety of
structures depending on the hybridization of the carbon acceptor.
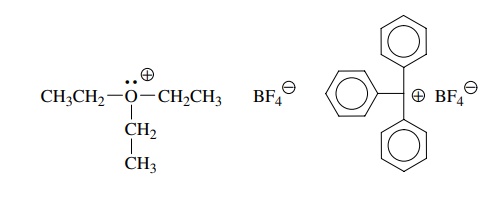
Trialkyloxonium tetrafluoroborates (Meerwein salts) R3O+ BF4−, for example, are
sp3-hybridized carbon elec-trophiles and are extremely reactive
toward nucleophiles. The acceptor orbital is an antibonding C–O σ ∗ orbital which is
low in energy because of the positive charge on oxygen.
Triphenylmethyl
(trityl) tetrafluoroborate, on the other hand, is sp2 hybridized but
it is also extremely reactive toward electron donors. The acceptor orbital of
the trityl cation is an unfilled 2p atomic orbital on the charged carbon. These
carbon electrophiles are isolable compounds, but they are extremely reactive
with any sort of electron donor (H2O vapor is a common culprit).
Many
other cationic carbon electrophiles cannot be isolated but can be gen-erated
insitu in a reaction mixture by Bronsted or Lewis acid–base reactions. In the
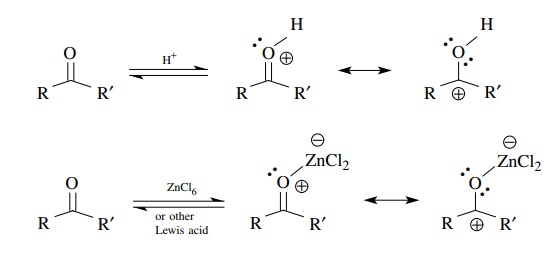
presence of Bronsted acids, carbonyl compounds are protonated and produce
positively charged oxonium ions. Compared to the carbonyl compound itself, the π ∗ orbitals of oxonium ions are much stronger electron
acceptors.
Protonation
of the carbonyl group is an equilibrium process, and the extent of protonation
(the position of the protonation equilibrium) is dictated by the pKa’s of the Bronsted acid and
the oxonium ion. While this equilibrium usually lies far to the left, the
reactivity of the oxonium ion is often sufficiently great that only small
amounts of the oxonium ion are needed to react effectively with the electron
donor.
Addition
of a strong Lewis acid such as TiCl4, BF3, or SnCl4
to a carbonyl compound is another common method to produce a very powerful
cationic elec-trophile in solution. Complexation between the Lewis acid and the
lone pairs of electrons on the carbonyl oxygen give a species which, although
formally neutral, behaves as a cationic carbon electrophile in the same fashion
as a proto-nated carbonyl group. These are strong electrophiles that react with
many types of nucleophiles. While aldehydes and ketones are common carbonyl
compo-nents which are activated with Lewis acids, esters and amides also yield
strongly electrophilic species with Lewis acids.
(b)
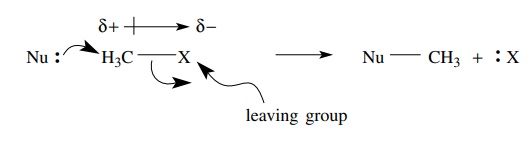
Aliphatic compounds with good leaving groups attached to primary or secondary
carbon atoms are very commonly used as carbon electrophiles. The leaving group
is an electronegative group attached by a polarized σ bond.
The
bond polarity makes the carbon atom electron deficient and capable of
accept-ing electrons from carbon electron donors (carbon-centered nucleophiles)
into the σ ∗ antibonding orbital. Population of the σ ∗ orbital by electron
donation weakens the bond to the leaving group. Ultimately the leaving group is
cleaved from the molecule and retains the pair of electrons from the connecting
bond. Examples of such compounds include alkyl halides, alkyl sulfonates, and
alkyl sulfates.
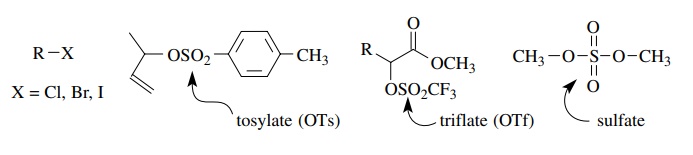
The
electrophilicity of such compounds is largely related to the leaving ability of
the leaving group. The leaving ability of a group is in turn related to (1) its
bond strength to carbon and (2) its ability to accept the bonded pair of
elec-trons and become electron rich (most often negatively charged). Leaving
abilities range from excellent (triflate) to moderate (chloride). The
electrophilicity of the acceptor molecule thus can be adjusted by changing the
leaving group.
(c)
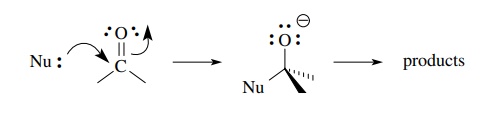
Carbonyl compounds are very common carbon electrophiles by virtue of the
polarized carbon–oxygen π bond.
Electron donation into the π ∗ orbital of the carbonyl carbon breaks the C–O π bond and produces a tetrahedral adduct
which can then proceed to products.
This
is a very general process for carbonyl compounds; however, the elec-trophilic
reactivity of the carbonyl group is very dependent on the groups attached to
it. The reactivity is ranked in the following order:
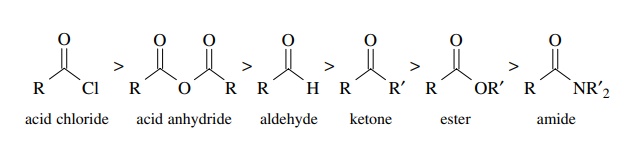
Electron-withdrawing
groups (Cl, RCO2) increase the electrophilicity while
resonance-donating groups –OR, –NR2 decrease the reactivity toward
electron donors. Steric effects are also a significant influence on carbonyl
reactivity. The trigonal carbonyl reactant goes to a more crowded tetrahedral
intermediate upon addition of the nucleophile; thus bulky groups attached to
the carbonyl carbon lead to more crowded transition states and result in much
slower addition reactions. This steric rationale is one explanation for the
greater reactivity of aldehydes over ketones. Extremely sterically hindered
ketones such as di-tert-butyl ketone
undergo carbonyl addition by nucleophiles at negligible rates for most
nucleophiles.
(d)
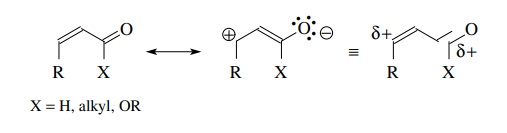
α,β-Unsaturated
carbonyl compounds can act as electrophiles under certain conditions and are
bidentate in that both the carbonyl carbon and the β carbon are electron deficient. Thus nucleophiles can attack at
either position.
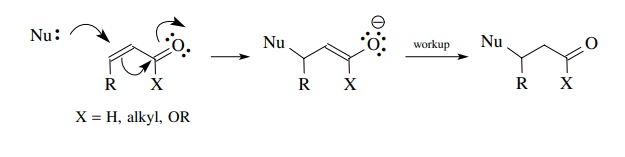
The
regioselectivity of nucleophilic addition is a function of the type of
nucleophile employed and in many instances can be controlled to give Michael
addition to the β carbon.
Related Topics
