Evaporation
| Home | | Pharmaceutical Technology |Chapter: Pharmaceutical Engineering: Evaporation and Distillation
Evaporation may be defined as the removal of a solvent from a solution by vaporization but is usually restricted to the concentration of solutions by boiling.
EVAPORATION
Evaporation
may be defined as the removal of a solvent from a solution by vaporization but
is usually restricted to the concentration of solutions by boiling.
Crystallization and drying, which may also utilize the vaporization of a
liquid, are considered in subsequent sections.
Evaporation
in the pharmaceutical industry is primarily associated with the removal by
boiling of water and other solvents in batch processes. However, the principles
that govern such processes apply more generally and are derived from a study of
the transfer of heat to the boiling liquid, the relevant physical properties of
the liquid, and the thermal stability of its components.
Heat Transfer to Boiling Liquids in an Evaporator
The
heat required to boil a liquid in an evaporator is usually transferred from a
heating fluid, such as steam or hot water, across the wall of a jacket or tube
in or around which the liquid boils. A qualitative discussion of the methods
used to secure high rates of heat flow can be based on equation (3.9):
Q = UAΔT
where
Q is the rate of heat flow, U is the overall heat transfer coefficient, A is
the area over which heat is transferred, and ΔT is the difference in temperature
between the fluids.
The
overall heat transfer coefficient is derived from a series of individual
coefficients that characterize the thermal barriers that oppose heat transfer.
Thus, for the heating fluid, the film coefficient for a condensing vapor, such
as steam, is high provided permanent gases and condensate are removed by
venting and draining. With liquid heating media, the velocity of flow over the
heat transfer surface should be as high as is practicable. If the solid barrier
consists of a thin metal wall, the resistance to heat flow will be small.
Resistance, however, is significantly increased by chemical scale, which may be
deposited on either side. The accumulation of scale should be prevented. A
glass wall may provide the largest thermal resistance of the system. Neglecting
the thermal stability of the boiling liquid, circulation of the liquid should
be rapid and, because of its influence on viscosity, the temperature of boiling
should be as high as possible. Both factors promote high film coefficients on
the product side of the wall.
Other
factors described by the above equation are the area of the heat transfer
surfaces, which should be as large as possible, and the temperature difference
between the heating surface and the boiling liquid. As long as the critical
heat flux is not exceeded, the latter should also be large.
The Physical Properties of Solution and Liquids
A
number of physical factors, which are interrelated in a complex way, are
relevant to a study of evaporation. For a given heating fluid, the temperature
difference across the wall of an evaporator is determined by the temperature of
boiling, a variable controlled by the external pressure and the concentration
of the solute in the solution. Both the boiling temperature and the solute
concen-tration influence the viscosity of the solution, a factor that greatly
affects the heat transfer coefficient. The temperature of boiling also
determines the solubility of dissolved constituents and the degree of
concentration that can be carried out without separation of solids.
The Relation Between Boiling Temperature and Solute Concentration
When
a solute is dissolved in a solvent, the vapor pressure is depressed and the
boiling point rises. Since the boiling point increases as the solute
concentration increases, the temperature difference between the boiling liquid
and the heating surface falls. For dilute solutions, the expected rise in
boiling point can be cal-culated from Raoult’s law. However, this procedure is
not applicable to con-centrated solutions or to solutions of uncertain
composition. For aqueous, concentrated solutions, Duhring’s rule may be used to
obtain the boiling point rise of a solution at any pressure. This rule states
that the boiling point of a given solution is a linear function of the boiling
point of water at the same pressure. A family of lines is required to cover a
range of concentration, as shown in Figure 10.1.
The Relation of Boiling Temperature and External Pressure
The
temperature at which a solution of given composition boils is determined by the
external pressure. The vapor pressure of a pure solvent at any temperature can
usually be obtained from published tables. Alternatively, if the vapor pressure
at two temperatures is known, the plot of the logarithm of the vapor pressure
against the reciprocal of the absolute temperature yields an approxi-mately straight
line. For intermediate pressures, the temperature at which the solvent will
boil can be found by interpolation. If dissolved substances are present, the
boiling point must be adjusted using Duhring’s rule. This value permits an
accurate estimate of the temperature differences in the evaporator.
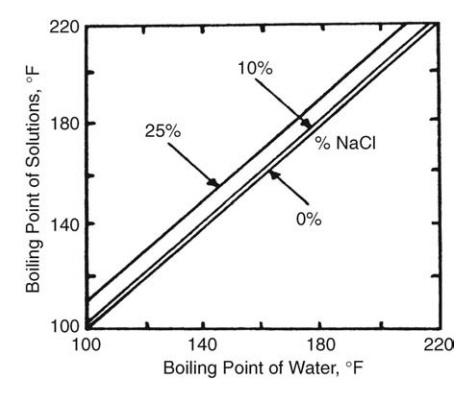
FIGURE 10.1
Duhring’s chart for sodium chloride.
Reduction
in the external pressure lowers the boiling temperature and, if the associated
increase in viscosity is not too great, increases the rate of evap-oration. On
large installations, a moderate vacuum is widely used to increase the capacity
of an evaporator. The imposition of low pressures and low boiling temperatures
is also necessary when thermolabile materials are processed.
Boiling
in tubes is commonly used in evaporators. In these circumstances, the
hydrostatic head developed by a column of liquid or the friction head imposed
by its movement can create a local increase in pressure, which sup-presses
boiling and decreases the evaporating capacity of the system.
The Relation of Viscosity with Temperature and Solute Concentration
The
viscosity of a solution is modified by changes in temperature and solute
concentration. Since a low viscosity promotes a high heat transfer coefficient,
the exponential decrease of viscosity with increase in temperature is of great
importance and indicates a high boiling temperature.
In
general, the addition of a nonvolatile solute increases the viscosity of a
solution at any temperature. Consequently, the viscosity of the solution
increases as the evaporation proceeds. These effects, however, cannot be
calculated.
If
at the operating temperatures and concentrations, the viscosity of a solution
is high, satisfactory heat transfer coefficients may only be obtained if the
liquid is driven over the heating surface. In other systems, movement of a
viscous liquid is assisted by gravity or the liquid in contact with the heating
surface is disrupted mechanically by scrapers.
The Effect of Temperature on Solubility
The
solubility of the components of a solution depends on the temperature. Most
commonly, solubility increases with increase in temperature, so a greater
degree of concentration is possible at higher temperatures without the
separa-tion of solids. The reverse is true for liquids containing scale-forming
solids with inverse solubility characteristics, such as calcium or magnesium
sulfate, or materials that decompose and deposit, such as coagulable protein.
The Effect of Heat on the Active Constituents of a Solution
The
thermal stability of components of a solution may determine the type of
evaporator to be used and the conditions of its operation. If a simple solution
contains a hydrolyzable material and the rate of its degradation during evaporation
depends on its concentration at any time, an exponential relation between the
remaining fraction, F, and the time, t, characteristic of a first-order
reaction, is obtained:
F
= e-kt (10:1)
The
dependence of the reaction velocity constant, k, on the absolute tem-perature,
T, is expressed by the relation
k = Ae-(B/T) (10:2)
where
A and B are constants characteristic of the reaction. Thus, at temperatures T1,
T2, and T3, where T1 > T2 > T3,
the relation between remaining fraction and time of heating shown in Figure
10.2 emerges. This indicates the importance of
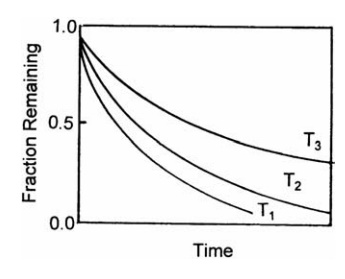
FIGURE 10.2 The effect of time and
temper-ature on degradation.
the
temperature and time of heating. If the latter can be shortened, the
temperature of evaporation can be greatly increased without increasing the
fraction that is degraded. If, therefore, the effect of temperature on the rate
of evaporation is known, it is possible to define conditions of time and
temperature at which decomposition is a minimum.
In
practice, the kinetics of degradation and the relation of evaporation rate and
temperature are usually not known. This is particularly true when the criteria
by which the product is judged are color, taste, and smell. In addition, the
analysis above neglects temperature variation in the evaporating liquid and
degradation in boundary films where temperatures are higher. Often, therefore,
experiments are necessary to determine the suitability of an evaporation
process.
In
batch processes, the time of exposure to heat is well defined. This is also
true for continuous processes in which the liquid to be evaporated is passed
only once through the heater. In continuous processes in which the liquid is
recirculated through the heater, the average residence time, a, is given by the
ratio
Working volume of
evaporato / Volumetric discharge
in
which volumetric discharge is only an indication of the damage that prolonged
heating may cause. If perfect mixing occurs in the evaporator, the fraction, f, which is in the unit for time, t, or less is given by the equation
f = 1- e-(t/a) (10:3)
This
relation shows, for example, that an evaporator with an average residence time
of one hour holds 13.5% of active principles for two hours and about 2% for
four hours.
Related Topics
