First-Order Half-Life
| Home | | Biopharmaceutics and Pharmacokinetics |Chapter: Biopharmaceutics and Pharmacokinetics : Pharmacokinetics Basic Considerations
the half-life of a first-order process is a constant and independent of initial drug concentration.
First-Order Half-Life
Substituting the value of C = Co/2 at t½
in equation 8.14 and solving it yields:
t1/2 = 0.693 / K (8.16)
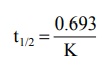
Equation 8.16 shows that, in contrast to zero-order
process, the half-life of a first-order process is a constant and independent
of initial drug concentration i.e. irrespective of what the initial drug
concentration is, the time required for the concentration to decrease by
one-half remains the same (see Fig. 8.4.). The t½ of a first-order
process is an important pharmacokinetic parameter.
Most pharmacokinetic processes viz. absorption, distribution and elimination follow first-order
kinetics.
Related Topics