Fluorinated pyrimidines
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Antifungal Agents
Fluorinated pyrimidines : Flucytosine - Synthesis and Drug Profile
Antifungal Agents - Synthesis
and Drug Profile
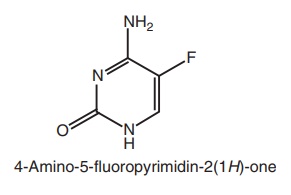
Fluorinated pyrimidines
1. Flucytosine
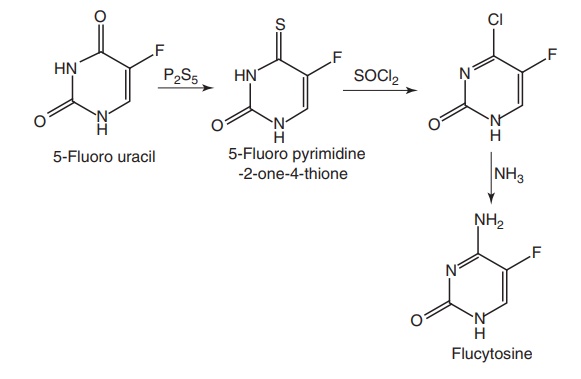
Synthesis
Route I. From: 5-Fluorouracil
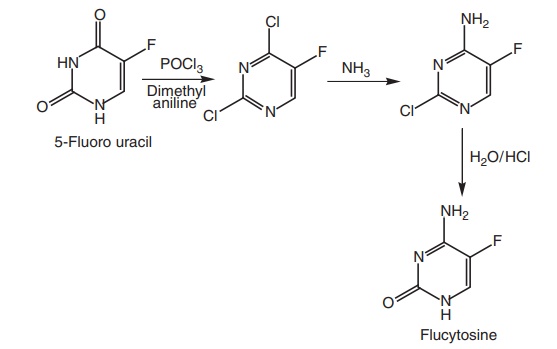
Route II. From: 5-Fluorouracil
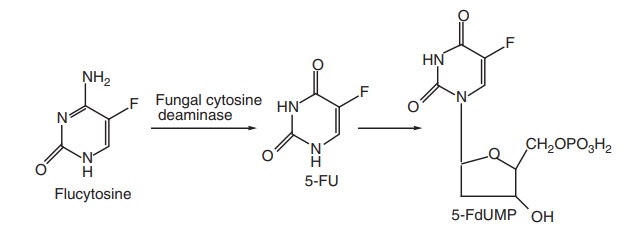
Mode of action: Flucytosine is converted by cytosine deaminase
into 5-flurouracil (5-FU), then, 5-fluoro deoxyuridylic acid is formed. This
false nucleotide inhibits thymidylate synthetase, thus, depriving the organism
of thymidylic acid, an essential DNA component. It is a potent antimetabolite,
which replaces uracil in the pyrimidine pool and thus, disrupts protein
synthesis. Mammalian cells do not convert flucytosine to fluorouracil. This fact
is crucial for the selective action of this compound. In addition,
5-fluorouracil is metabolized into 5-fluoro uridylic acid by the enzyme uridine
monophosphate (UMP) pyrophosphorylase. It is either incorporated into the DNA
(via synthesis of 5-fluorouridine triphosphate) or would be metabolized into
5-fluoro deoxyuridylic acid, which is a potent inhibitor of thymidylate
synthetase.
Metabolism: It is metabolized to 5-FU by fungal cytidine deaminase.
Then 5-FU is
converted into 5-fluorodeoxyuridine, which is a thymidylate synthase inhibitor
and interferes with both protein and RNA biosynthesis.
Properties and uses: Flucytosine is a white crystalline powder,
sparingly soluble in water, and slightly soluble in ethanol. Flucytosine is the
only available antimetabolite drug having antifungal activity.
Assay: Dissolve the sample in anhydrous acetic acid, add acetic
anhydride, and titrate with 0.1 M perchloric acid. Determine the end point
potentiometrically.
Dosage forms: Flucytosine tablets B.P.
Related Topics
