Formation of Urine
| Home | | Anatomy and Physiology | | Anatomy and Physiology Health Education (APHE) |Chapter: Anatomy and Physiology for Health Professionals: Urinary System
The purposes of urine formation are to cleanse the blood and balance the body’s chemical substances. Three steps are involved in urine formation and the regulation of blood composition: glomerular filtra-tion, tubular reabsorption, and tubular secretion.
Formation of Urine
The purposes of urine formation are to cleanse the blood and balance the body’s chemical substances. Three steps are involved in urine formation and the regulation of blood composition: glomerular filtration, tubular reabsorption, and tubular secretion.
Glomerular Filtration
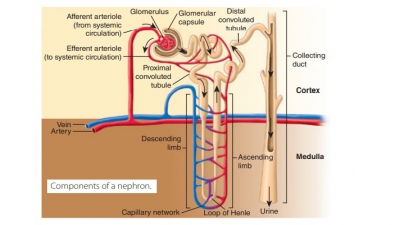
Glomerular filtration is a passive process that ini-tiates urine formation in the renal corpuscle. It uses hydrostatic pressure to force fluids and solutes through membranes. The plasma is filtered by the glomeru-lar capillaries, producing filtrate that is free of cells and proteins. Most of this fluid is reabsorbed into the bloodstream via the colloid osmotic pressure of the plasma. Using two capillaries in series, the neph-rons use glomerular filtration to help produce urine. The first capillary bed filters forming interstitial fluid but does not form it. The filtrate, which is derived from the plasma, moves into the renal tubule to form urine. Every 24 hours, glomerular filtration produces 180 liters or 47 gallons of fluid—this is more than four times the amount of total body water. Less than 1.5 liters, which is lower than 1% of the fluid, leaves the body as urine.
The filtration membrane is located between the interior glomerular capsule, also called Bowman’s capsule, and the blood. It is a porous membrane, allowing water and solutes that are smaller than plasma proteins to freely pass through. Any mac-romolecules caught in the filtration membrane are engulfed by glomerular mesangial cells, which are spe-cialized pericytes. Any molecules smaller than 3 nm in diameter can pass through freely, from the blood into the capsule. These molecules include amino acids, glucose, nitrogenous wastes, and water. An example of a substance that is too large to pass through the filtra-tion membrane is albumin.
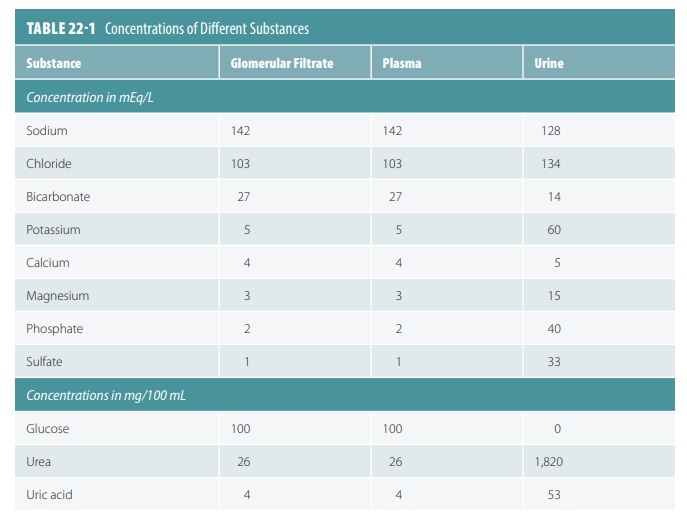
Because of this, these substances are similar in concentration to blood and glomerular filtrate. Glo-merular filtrate is mostly water with the same com-ponents as blood plasma, except for large protein molecules. TABLE 22-1 compares concentrations of substances in glomerular filtrate, plasma, and urine. Molecules larger than 5 nm usually cannot enter the tubules, so plasma proteins remaining in the capillar-ies help to regulate the oncotic or colloid osmotic pres-sure of glomerular blood. Therefore, all water cannot be lost to the capsular spaces. The filtration membrane is probably functioning abnormally when proteins or blood cells become present in the urine.
The outward pressure that affects filtration is the hydrostatic pressure in the glomerular capillaries. This is basically the same as glomerular blood pressure and the strongest force moving water and solutes from the blood across the filtration membrane. Here, the blood pressure is high in the glomerulus, at about 55 mm Hg. In other capillary beds, the pressure is only 26 mm Hg.
The reason for the difference is that the glomerular cap-illaries drain via a high-resistance efferent arteriole with a smaller diameter than the afferent arteriole supplying them. Filtration then occurs along all the length of every glomerular capillary. Reabsorption does not occur as it does in other capillary beds. The colloid osmotic pressure in the glomerular capsular space is nearly zero, because practically no proteins enter the capsule.
The osmolarity of a solution is its osmotic concen-tration or the total number of solute particles per liter. This is usually expressed in osmoles per liter (Osm/L) or milliosmoles per liter (mOsm/L). Body fluids have an osmolarity of approximately 300 mOsm/L, whereas fresh water is 5 mOsm/L and seawater 1,000 mOsm/L.
Glomerular Filtration Rate
The glomerular filtration rate (GFR)is defined as the volume of filtrate that forms every minute by the activ-ities of the two million glomeruli in the kidneys. It is directly proportional to the net filtrationpressure, the total surface area that is available for filtration, and the permeability of the filtration membrane (FIGURE 22-7). The net filtration pressure forces sub-stances out of the glomerulus and is usually positive pressure. Tiny openings called fenestrae in glomer-ular capillary walls make them more permeable than the capillaries of other tissues. Cells called podocytes cover these capillaries, helping to make them imperme-able to plasma proteins. The feet of the podocytes are called pedicels. Materials that pass from the blood, at the glomerulus, must be tiny enough to move through filtration slits between adjacent pedicels.
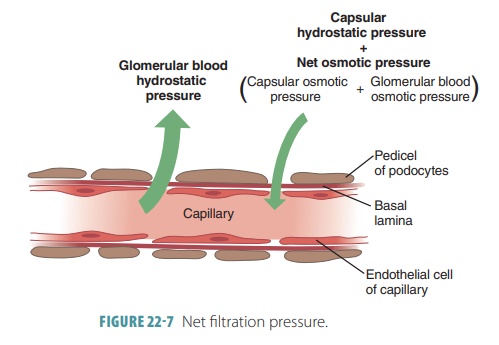
Net filtration pressure is directly proportional to the GFR. All factors related to GFR affect net filtration pressure. Any changes in afferent or efferent arteriole diameters alter the GFR. During filtration through capillary walls, proteins in the plasma raise colloid osmotic pressure with the glomerular capillaries. Anything that decreases plasma colloid osmotic pres-sure increases the filtration rate. The kidneys consume up to 25% of all oxygen the body uses while resting.
Regulation of Glomerular Filtration
The GFR is regulated by intrinsic controls or renal auto-regulation and extrinsic controls via the nervous and endocrine systems. The intrinsic controls occur within the kidneys, whereas the extrinsic controls maintain blood pressure. The GFR must be relatively constant, so the kidneys can manufacture filtrate and maintain extracellular homeostasis. The body’s overall blood pressure must also be relatively constant. If nothing else changes, when the GFR increases, urine output also increases. This reduces blood volume and blood pres-sure. When the GFR decreases, the opposite occurs.
Extreme changes to mean arterial pressure, less than 80 mm Hg or higher than 180 mm Hg, causes the extrinsic controls to dominate to stop damage to the brain and other vital organs. Changing only the glomerular hydrostatic pressure can control the GFR. Therefore, the major mechanisms of pressure control focus on changing the glomerular hydro-static pressure. When it rises, so do both the net filtration pressure and the GFR. The GFR will be reduced to zero if the glomerular hydrostatic pres-sure falls by 18% or greater, meaning it must be con-trolled precisely.
The GFR is kept relatively constant by autoregu-latory mechanisms over the 80–180 mm Hg arterial pressure range. Normal daily changes in exercise activity, posture, or sleep do not result in significant changes in excretion of water and solutes. However, serious hemorrhage that causes extremely low sys-temic blood pressure will result in hypovolemic shock, which cannot be stopped by the intrinsic controls.
Therefore, when mean arterial pressure is lower than 80 mm Hg, extrinsic controls take over and the auto-regulatory mechanisms stop working.
The hormonal and neural mechanisms used in extrinsic controls include the sympathetic nervous system and the renin–angiotensin–aldosterone mech-anism. Although the neural kidney controls help the whole body, the kidneys may suffer as a result. The kid-ney blood vessels are dilated, with their autoregulatory mechanisms dominating control when extracellular fluid volume is normal. At this time, the sympathetic nervous system is resting. Once the extracellular fluid volume becomes lower than 80 mm Hg, blood must be moved or shunted to vital organs. At that point, neural controls override autoregulatory mechanisms, which may reduce kidney blood flow enough to dam-age these organs.
Falling blood pressure causes the sympathetic nerve fibers to release norepinephrine and the adrenal medulla to release epinephrine. As a result, vascular smooth muscles constrict. This increases periph-eral resistance, returning blood pressure to normal. This is the baroreceptor reflex. The GFR decreases from constriction of the afferent arterioles, assist-ing blood volume and pressure to return to normal. The renin–angiotensin–aldosterone mechanism is the primary control that increases blood pressure, yet it regulates GFR indirectly. Inadequate blood pressure means glomerular filtration cannot occur. It causes the granular cells in the juxtaglomerular complex to release renin. This occurs by either direct stimulation of granular cells, stimulation of granular cells via input from activated cells of the macula densa, or reduced stretching of granular cells.
Secretion of renin responds to three types of stimuli:
■■ When special afferent arteriole cells sense a drop in blood pressure
■■ In response to sympathetic stimulation
■■ When the macula densa senses decreased chloride, potassium, and sodium ions that reach the distal tubule
Renin in the bloodstream reacts with the plasma protein angiotensinogen, forming angiotensin I. In the lungs and blood plasma, angiotensin- converting enzyme converts angiotensin I to angiotensin II. Angiotensin II helps maintain sodium and water balance as well as blood pressure. It vaso-constricts the efferent arteriole, raising glomerular capillary hydrostatic pressure. This helps the decrease in GFR. Angiotensin II stimulates the adrenal cortex to secrete aldosterone, encouraging tubular reabsorp-tion of sodium.
1. Explain which types of molecule are trapped by the glomerular filtration membrane and which ones are not.
2. Contrast how glomerular filtration is regulated by intrinsic and extrinsic controls.
3. Describe the two types of extrinsic controls involved in glomerular filtration.
Tubular Reabsorption
Two other processes contribute to urine formation. Tubular reabsorption selectively moves substances from the tubular fluid into the blood within the per-itubular capillary (FIGURE 22-8). This occurs in the renal tubules and collecting ducts and is a transepithe-lial process. The kidney reclaims the correct amounts of water, electrolytes, and glucose as required by the body. All amino acids and glucose that were filtered are reabsorbed. Approximately, 99% of water, sodium, and components are also reabsorbed. What is left over becomes urine.
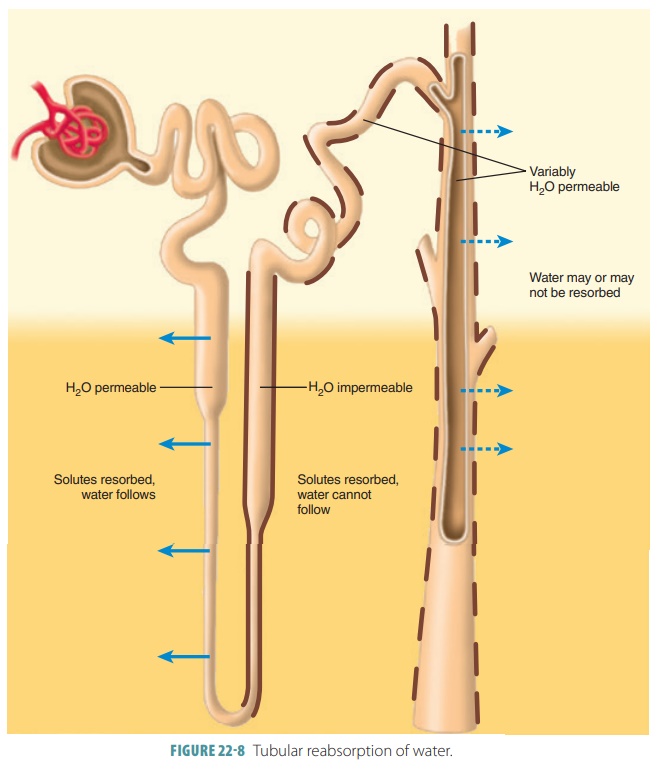
Tubular reabsorption begins as soon as filtrate enters the proximal tubules. If not for tubular reab-sorption, all the body’s plasma would drain out as urine in under 30 minutes. In healthy adults, the total plasma volume filters into the renal tubules nearly every 22 minutes. Reabsorbed substances use either a paracellular or transcellular route to reach the blood. The paracellular route between the tubule cells is less extensive because tight junctions connect these cells. These tight junctions are, however, not as tight in the proximal nephron and allow water and certain ions to pass through via the paracellular route. These ions include calcium, magnesium, potassium, and less amounts of sodium.
Nearly all organic nutrients such as glucose or amino acids are totally reabsorbed by healthy kid-neys. This can maintain or restore normal plasma concentrations. However, water and specific ion reab-sorption is regularly controlled as a result of signals from hormones. The reabsorption process is either active or passive, based on the actual substances trans-ported. Adenosine triphosphate is required for active tubular reabsorption. This may be direct, as in primary active transport, or indirect, as in second-ary active transport. The process of passive tubular reabsorption uses diffusion, facilitated diffusion, and osmosis. In these processes, substances move down their own electrochemical gradients.
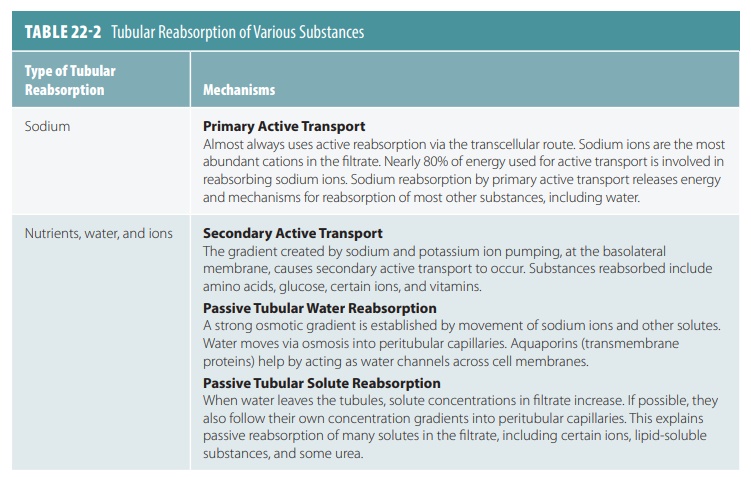
Substances remaining in the renal tubule become more concentrated as water from the filtrate is reab-sorbed by osmosis. Water reabsorption increases if sodium reabsorption increases and vice versa. Active transport reabsorbs nearly 70% of sodium ions in the proximal renal tubule. Passive, negatively charged ions move along with the sodium ions. This is a form of passive transport because it does not require cel-lular energy expenditure. Movement of solutes and water into the peritubular capillary reduces fluid vol-ume inside the renal tubule. Active transport reab-sorbs sodium ions continually as tubular fluid moves through the nephron loop, distal convoluted tubule, and collecting duct. Nearly all sodium ions and water from the renal tubule via the glomerular filtrate are reabsorbed before urine excretion. Tubular reabsorp-tion of sodium, nutrients, water, and ions is explained in TABLE 22-2.
All the renal tubule is involved in reabsorption in varying degrees. However, the proximal convoluted tubule cells are the most active in this regard. These cells normally reabsorb all amino acids and glucose in the filtrate. They also normally absorb 65% of the water and sodium in the filtrate. By the time filtrate reaches the nephron loop, most electrolytes have been reabsorbed. In the proximal tubule, almost all uric acid and about 50% of urea are reabsorbed. However, both substances are eventually secreted back into the filtrate.
Once reaching the nephron loop, permeability of tubule epithelia changes greatly. At this point, water reabsorption is no longer related to solute reabsorp-tion. Although water can leave the nephron loop’s descending limb, it cannot leave the ascending limb. This is where the aquaporins are at very low levels or absent in the tubule cell membranes. These differences in permeability are vitally important parts of how the kidneys form urine that is either concentrated or dilute. For solutes, a reverse situation compared with that of the water is true. They leave the ascend-ing limb of the nephron loop but not the descending limb. Nearly no reabsorption of solutes occurs in the descending limb, yet they are both actively and pas-sively reabsorbed in the ascending limb.
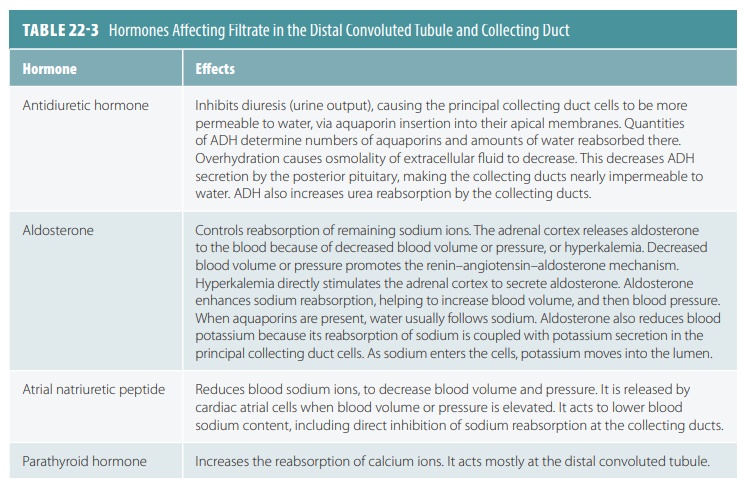
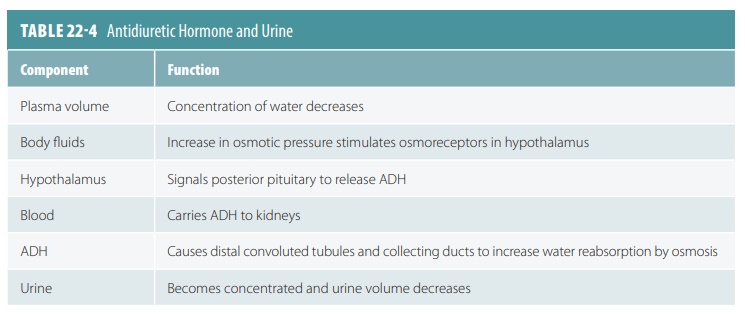
Reabsorption in the distal convoluted tubule and collecting duct is different. It is adjusted by hormones. Because most filtered water and solutes have already been reabsorbed, only a small part of the filtrate is affected. Approximately, 10% of originally filtered sodium chloride and 25% of water experience hor-monal adjustment. The hormones that are involved include antidiuretic hormone (ADH), aldosterone, atrial natriuretic peptide, and parathyroid hormone. These hormones are explained in greater detail in TABLES 22-3 and 22-4 summarizes how ADH helps reg-ulate urine concentration and volume.
Tubular Secretion
Tubular secretion selectively moves substances from the blood in the peritubular capillary via the filtrate into the renal tubule. These substances include hydro-gen, ammonia, potassium ions, creatinine, and various organic acids and bases. Certain substances synthe-sized in the tubule cells such as bicarbonate are also secreted. Although essentially the opposite of tubular reabsorption, it also occurs along the length of the renal tubule and collecting duct. Some substances that the body must excrete, such as hydrogen ions and some toxins, are removed more quickly than through filtration. The proximal convoluted tubule is the main site of excretion, except for potassium. The cortical sec-tions of the collecting ducts are also active in secretion.
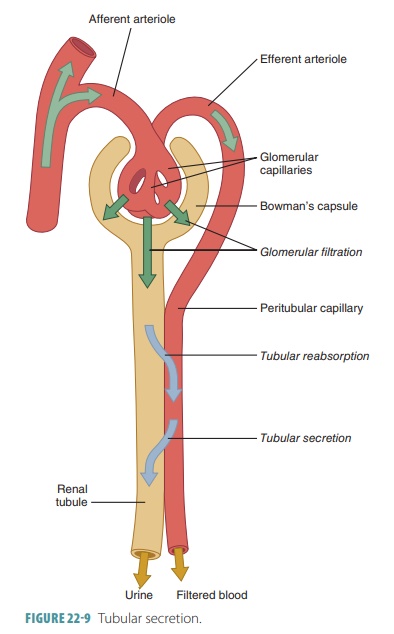
Tubular secretion is important for four major pro-cesses: disposing of substances, eliminating undesirable substances, removing excessive potassium, and con-trolling blood pH. Tubular secretion disposes of cer-tain drugs and metabolites that may be tightly bound to plasma proteins. Because these proteins are usually not filtered, the bound substances are also not filtered, and therefore must be secreted. Tubular secretion eliminates undesirable substances or end products that have been passively reabsorbed (FIGURE 22-9). Exam-ples include the nitrogenous wastes, and also urea and uric acid. Nephron processing of urea is complicated, but basically up to 50% of filtrate urea is excreted.
Tubular secretion also removes excessive potas-sium from the body. Nearly all potassium ions in the filtrate are reabsorbed in the proximal convoluted tubule and ascending nephron loop. Therefore, almost all potassium in the urine is derived from active tubu-lar secretion into the last portions of the distal con-voluted tubule and collecting ducts. This secretion is controlled by aldosterone. When blood pH drops toward being acidic, renal tubule cells actively secrete more hydrogen ions into the filtrate. They retain and generate more bicarbonate, which is a base. There-fore, blood pH rises and the urine drains off any excess hydrogen. In the opposite situation, blood pH approaching alkalinity causes chloride ions to be reab-sorbed instead of bicarbonate. The bicarbonate then leaves the body via the urine.
At the distal convoluted tubule, potassium ions are removed from the peritubular fluid in exchange for sodium ions from the tubular fluid. The potas-sium ions then diffuse through potassium leak channels. Hydrogen ions generated by carbonic acid dissociation are secreted in exchange for sodium ions in the tubular fluid. This acidifies the tubular fluid while blood pH is elevated. Because production of lactic acid and ketone bodies during postabsorp-tion can cause acidosis, both the proximal and distal convoluted tubules deaminate amino acids to strip off the amino groups. The reaction sequence binds hydrogen ions and yields ammonium and bicarbon-ate ions. The ammonium ions are pumped into the tubular fluid, whereas the bicarbonate ions enter the bloodstream via the peritubular fluid. There-fore, tubular deamination provides carbon chains for catabolism while generating bicarbonate ions to increase the plasma’s buffering capacity.
Function of the Vasa Recta
The vasa recta functions to return solutes and water reabsorbed in the renal medulla to the bloodstream without changing the concentration gradient. These long, straight capillaries are parallel to the long nephron loop of the juxtamedullary nephrons. When blood enters the vasa recta from the peritu-bular capillaries, its osmotic concentration is nearly 300 mOsm per liter. Blood descending into the medulla gradually increases in osmotic concentra-tion when the peritubular fluid’s solute concentra-tion rises. This increase utilizes solute absorption as well as water loss. Solute absorption is the main fac-tor since plasma proteins control the osmotic flow of water from the blood.
Blood that ascends toward the cortex slowly decreases its osmotic concentration, as there is a decline in solute concentration of the peritubular fluid. This also involves both solute diffusion and osmosis. However, osmosis is the main factor here, since the presence of plasma proteins has no opposition to the osmotic flow of water into the blood. Overall, the results inside the vasa recta are:
■■ Some solutes absorbed in the descending portiondo not diffuse out in its ascending portion
■■ More water moves into the ascending portion than moves out in the descending portion
Therefore, the vasa recta carries water as well as sol-utes out of the medulla. Normally, removal of solutes and water by the vasa recta allows for precise bal-ancing of solute reabsorption and osmosis inside the medulla.
1. Where does most reabsorption occur in the nephron?
2. Contrast the primary and secondary active transport processes.
3. Explain how movement of sodium ions controls reabsorption of water and solutes.