Formulation of Toothpastes
| Home | | Pharmacy Cosmetic Formulation |Chapter: Textbook of Cosmetic Formulation : Toothpastes
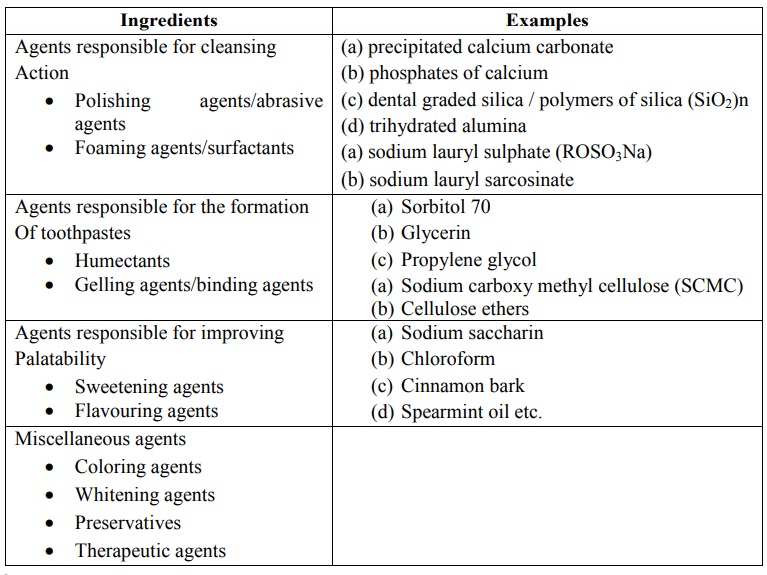
Toothpastes are the most popular form of dentifrices. They include the following ingredients which determine the quality and efficiency of toothpastes.
FORMULATION
Toothpastes are the most popular
form of dentifrices. They include the following ingredients which determine the
quality and efficiency of toothpastes.
1.
Polishing Agents / Abrasive Agents:
The
abrasives or the polishing agents are used to polish the teeth and remove food debris adhered to the surface of
the teeth. They are used in concentration of about 20 - 50% of the total
formulation.
They should possess the following
characteristics:
Ingredients Examples
The most commonly used Abrasive
agents are as follow:
(a)
Precipitated Calcium Carbonate
(CaCO3): It is also
known as precipitated chalk and is available
in a number of grades. The crystalline form of the precipitated chalk may be
available as:
(i)
Calcite:
Contains rhombohedral crystals.
Aragonite: Contains orthorhombic
crystals.
Advantages:
·
It
is of very low cost.
·
It
is available in different grades in white or off-white colours.
·
The
lighter grades are very stable and do not get hardened on storage.
Disadvantages:
·
The
abrasivity is not consistent within the lots of same grade of powder due to the
presence of impurities.
·
It
is incompatible with sodium fluoride which is used as anticaries agent.
(b)
Phosphates of Calcium: A large variety of insoluble calcium
phosphates are used as abrasive
agents. They may he as follows:
Dicalcium Phosphate (DCP) Dihydrate [CaHPO4.2H2O
]: It is a commonly used abrasive agent among the phosphate of calcium.
Its properties and the advantages and disadvantages are follows.
Advantages:
·
It
provides good flavour stability.
·
Toothpastes
made with Dicalcium phosphate are better than toothpastes made with chalk.
·
They
do not make use of additional whitening agents.
·
The
hardening of the paste during preparation is accelerated in the presence-of
fluoride ions.
·
It
has less abrasive effect on dentine.
Disadvantages:
·
It
is incompatible with sodium fluoride.
·
The
only source of fluoride is sodium monoiluoro phosphate since it consists of
free calcium ions that react with other fluoride sources leading to
incompatibility.
·
The
DCP Dihydrate is unstable in its natural for and may convert into anhydrous
form which may result in hardening of the paste.
The other commonly used phosphates
of calcium are tricalcium phosphate, calcium pyrophosphate etc., The insoluble
sodium metaphosphate, dibasic ammonium phosphate are also used as abrasive
agents.
(c)
Dental grade silica / Polymers of
Silica (SiO2)n : They are polymer of silica that are commonly used as abrasive agents in the formulation of toothpaste
`gels in large quantities. They are available in two forms as:
·
Abrasive
Form of Silica.
·
Thickening
Form of Silica.
Abrasive Silica: They are also referred to as xerolgels. They possess good
abrasive property and are used in
low concentration. They have least effect on the consistency of the finished
product.
Thickening silica: They are referred to as aerogels. The particles are small in
size and posses a greater surface
area. They have the ability to swell and provide a thickening effect to the pastes.
Advantages:
The silicas are mostly used as abrasives
in gels.
They are inert and easily compatible
with other ingredients.
They provide good gloss to the
dentine due to their high refractive index.
They can be used in low concentration.
Disadvantage:
The abrasive property is not consistent
within the different grades.
(d)
Trihydrated Alumina (Al2O3.3H2O):
It may be available in two forms: As
suspension or as crystalline powder.
Advantages:
·
It
is less costly.
·
It
possesses stability with fluorides.
·
It
is easily available and is stable during storage.
·
It
is compatible with other ingredients.
·
It
possesses a good abrasive property.
Disadvantage:
It has poor thickening property.
2.
Foaming Agents / Surfactants:
They
are also known as wetting agents. The mechanism of cleansing action is by reducing the surface tension at the interface
of the adhered material and enamel of the teeth.
They aid in abrasive action by
wetting the surface of the teeth. They help in the diffusion of into narrow
spaces, thus enhancing the cleansing action. The properties of the surfactants
are as follows:
·
It
should be compatible with other ingredients of the formulation.
·
It
should possess good surface active property.
·
It
should be non-toxic and non-irritant to the oral mucosa of the buccal cavity.
·
It
should be tasteless.
The most commonly used surfactants
are:
(a)
Sodium Lauryl Sulphate (ROSO3
Na): It is used
in concentrations of 0.5 to 2% in order to
provide necessary foaming action.
Advantages:
·
It
is available in a large variety of graded forms.
·
The
recrystallized grades have good surfactant property.
·
They
are more compatible with other ingredients of the formulation.
·
They
have a neutral pH range.
Disadvantages:
·
The
nature of the foaming agent may be altered by the presence of any free alcohol
content.
·
The
different grades are very expensive.
(b)
Sodium Lauryl Sarcosinate: It is one of the most preferred
detergents for oral products.
Advantages:
·
It
shows anti-enzymatic activity besides acting as a surface active agent.
·
It
is easily soluble in aqueous solvents and hence most preferred for the formulation
of oral products.
·
It
is consistently stable with a neutral pH range.
Disadvantage:
It may alter the taste of the final
formulation when used in high concentrations.
3.
Humectants:
Humectants
are used in order to prevent the rapid drying of dentifrices. They prevent excessive moisture loss from
the product. They may additionally impart plasticity to the final product. The
concentration of the humectant used in the formulation may vary from 20% to
40%.
The most commonly used humectants in
the formulation of dentifrices are as follows:
(a)
Sorbitol 70: It consists of 70% w/v concentration
of the sorbitol solution. It comprises the
largest pan the humectant phase.
Advantages:
·
It
has high viscosity and can produce firm toothpastes with good plasticity.
·
It
imparts cool sensation in the mouth and may also enhance the sweetening
property.
It possesses good compatibility with
other ingredients; it is less expensive than glycerin.
(b)
Glycerine: It can be used at concentrations ranging
between 5 to 10%.
Advantages:
·
It
provides a good gloss and good shine to the product.
·
It
is safe, stable and compatible with other ingredients.
·
It
is easily available both from natural and synthetic sources.
Disadvantages:
·
It
is very expensive.
·
It
provides a warm sensation in the mouth.
(c)
Propylene Glycol: it is less commonly used and has
been replaced by sorbitol.
Advantage:
It has good solvent properly and can
also be used as a co-solvent.
Disadvantage:
It has very low viscosity and may also
impart a bitter taste to the product.
4.
Ceiling/ Binding Agents:
The
binding agents are used in order to hold the solid and the liquid components together to form a smooth paste and maintain its
property, particularly during storage. They prevent bleeding from the paste and
also add up to the body and viscosity of the final formulation.
The commonly used binding agents are
cellulose derivatives such as Carboxy Methyl Cellulose (CMC), Sodium Carboxy
Methyl Cellulose (SCMC), HydroxyethyJ cellulose, Cellulose ethers etc.
(a)
Sodium CMC: It is a commonly used cellulose
derivative and used in concentrations between
0.9 to 2.0%. It is sensitive to pH value outside 5.5 to 9.5. The properties
with its advantages and disadvantages are as follows:
Advantages:
·
It
provides stability to the gels.
·
It
resists change in the efficiency of the formulation even in the presence of
divalent calcium ions and other electrolytes.
Disadvantage:
It may react with cationic
substitutes of antibacterial agents due to its anionic nature. Hence it cannot be used in such formulations.
(b)
Ethers of Cellulose: Methyl cellulose and
hydroxyethylcellulose are the most commonly
used cellulose ethers.
Advantages:
·
They
are stable over a wide range of pH changes.
·
They
are not affected by the metallic ions.
·
They
can be used in the toothpastes containing cationic antibacterials.
·
The
properties can be adjusted as required by varying the degree of substitution of
the components.
Disadvantages:
·
The
toothpastes made with cellulose ethers are more viscous at and stiff and
disperse slower than those made with SCMC.
·
They
cannot be used with glycerine as they are incompatible with it.
The other naturally available
gelling agents may be Gum karaya, Gum tragacanth, Iris moss (Chondrus), Gum
Arabica etc,
(c)
Water: Water is used in the deionized form
in the formulation of toothpastes. It can be used either as a solvent for the soluble ingredient of the
formulation or as a supporting media for the binding agents. Binding agents
swell after imbibing water. It is used in concentrations of more than 10% in
the formulation of clear gels.
5.
Sweetening Agents:
These
are added in. order to improve the sweetening properties and cover the bitter taste of the other
ingredients like surfactants, binders etc. They help in promoting the
acceptance of the product when administered orally.
The most commonly used sweetening
agents are Saccharin sodium, Chloroform, Aspartame, Cyclamates and Potassium
acesulfame.
(a)
Saccharin Sodium: It is the most widely used
sweetening agent. It is used at concentrations
of about 0.05 0.3 1 %. The concentration may vary depending upon the amount of
humectant (glycerine) used.
Advantages:
·
It
is of low cost.
·
It
is widely distributed and easily available.
·
It
is compatible with all other ingredients.
·
It
provides good sweetening property.
(b)
Chloroform:
Advantages:
·
It
masks the taste of precipitated chalk and prevents dry feeling in the mouth.
·
It
provides a fresh and sharp sweetness.
·
It
also has antibacterial property besides the sweetening property.
Disadvantages:
·
It
is expensive.
·
It
is incompatible with certain ingredients.
6.
Flavouring Agents:
Flavouring agents may comprise the most proprietary and most crucial part of the formulation
essential to meet the consumer preferences. They are generally a mixture of
edible volatile oils consisting of spearmint and peppermint oil as major
components. The other components included may be thymol, anethol, eucalyptol,
aniseed oil, oil of winter green etc. Flavouring agents are used in the
concentration range of about 0.5 to 1.
5% and constitute the most costly part of the formula; they may interact
with other components of the formulation which may result in incompatible.
7.
Colouring Agents:
They
are used in concentration of less than 0.01% as permitted by the EEC Cosmetics Directive. They can be
used generally in combination with a portion of a white creamy base. They are
mainly in order to influence consumer preferences and increase the purchase
intent.
8.
Whitening Agents:
Whitening agents such as Titanium
dioxide (TiO2) shall be preferentially
added in order to provide additional whiteness and brilliance to the paste.
9.
Preservatives:
Preservatives
are used in the formulation in order to maintain the properties of the product throughout the storage
period and to improve the shelf-life of the product. Generally, a mixture of 5%
methyl paraben and 0.02% propyl paraben is the most effective and commonly used
combination preservatives. Sodium benzoate is not preferred due to its
incompatibility with some of the therapeutic agents.
10.
Therapeutic Agents:
Therapeutic agents are included in
toothpastes in order to provide additional
beneficial effects besides normal cleansing properties.
Examples:
(a) Anticaries Agents:
Example:
Fluoride derivatives like NaF, Na2FPO3, etc,
(b) Antiplaque Agents:
Example:
Chlorohexidine, Triclosan etc,.
(c) Antitartar Agents that prevent the
Colouring of Teeth :
Example:
Zn salts, Pyrophosphate ions, Tetra sodium pyrophosphate, Disodium
dihydrophosphate.
(d) Sensitive Dentine Agents:
Example: Strontium
chloride, Strontium acetate, Formaldehyde etc,.
(e)
Optical Brightness:
Example: Substituted
coumarins in long
chain alkylamines.
(e)
Bleaching Agents:
Example:
H 2 O 2 , Sodium peroxide.
(g)
pH Regulators:
Example:
Zirconium silicate.
Toothpaste
Formula:
Formula-1 Quantity for 100 g
Calcium
carbonate (adhesive agent) 28 g
sodium
lauryl sulphate (surfactant) 0.5 g
Glycerin
(humectants) 11 g
Gum
tragacanth (binding agent) 0.75 g
Water
(liquid phase) 9.7 g
Saccharin
sodium (sweetening agent) 0.05 g
Flavor
(flavoring agent) q. s
Preservative
(for storage) q. s
