Fragmentation Processes
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Structure Determination of Organic Compounds
Besides the molecular ion, fragmentation processes can be used to infer groups present in the molecule and the connectivity of those groups.
FRAGMENTATION PROCESSES
Besides
the molecular ion, fragmentation processes can be used to infer groups present
in the molecule and the connectivity of those groups. The requirement of spin
and charge conservation in any fragmentation means that both cations and
radical cations can be produced as ions by fragmentation. Because of the great
amount of energy deposited in the molecular ion, there is sufficient energy to
break any of the bonds in the molecule. It has been found, however, that
fragmentations tend not to be random but occur in such a way that the most
stable ions are produced. Normally the most stable ion is the most abundant ion
in the mass spectrum. The most abundant ion is called the base peak of the spectrum and is arbitrarily scaled at 100%, and
the abundances of other ions are given as percentage relative to the base peak.
Several examples of very stable ions are as follows:
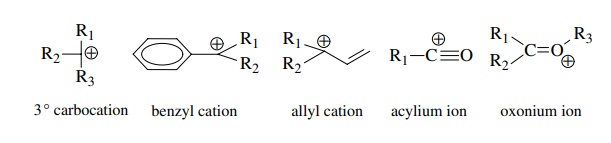
Fragmentations
often occur from the molecular ion by loss of neutrals or rad-icals to give
more stable ions or radical ions. The differences in mass correspond to the
mass of the uncharged fragment that has been expelled. The mass spectrum of
ethane has a molecular ion at m/e = 30 and a major peak at m/e = 15. This corresponds to the loss of
a fragment of 15 amu from the molecular ion. Thus the ethane molecular ion
undergoes fragmentation of the C–C bond to give a methyl cation which is
detected at m/e = 15 and a methyl radical which is
not detected as it is uncharged. This very simple example is indicative of the
process.
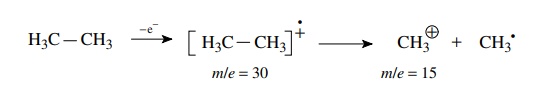
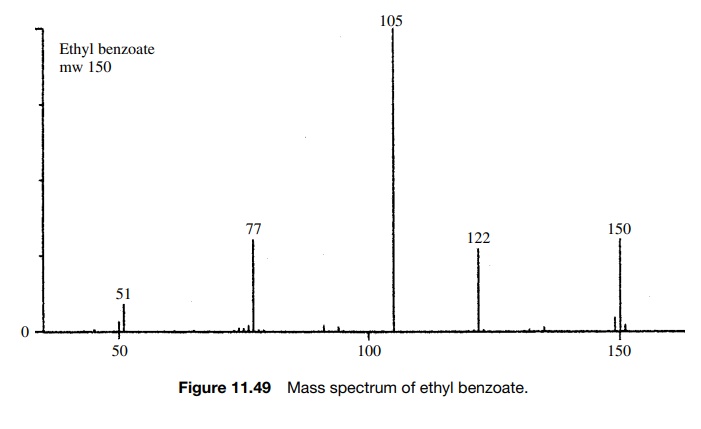
Ethyl
benzoate (Figure 11.49) has a molecular ion at m/e = 150 and a base peak
at m/e = 105 (M − 45) and a smaller peak at m/e = 77. The base peak at m/e = 105 corresponds to loss of the
ethoxy radical from the molecular ion
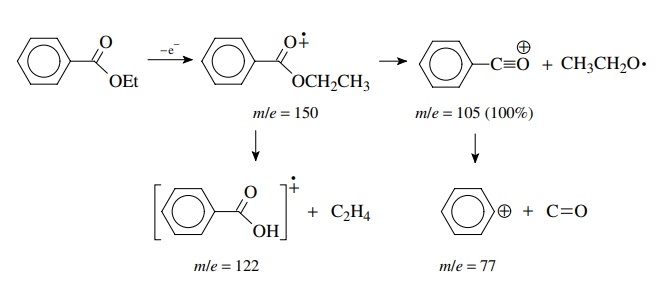
Loss of CO from the phenylacylium ion
gives the phenyl cation, but due to the instability of the phenyl cation, this
pathway is minor. Also observed is a peak at m/e = 122 due to the
benzoic acid radical cation resulting from loss of the neutral ethylene
molecule from the molecular ion by a different fragmentation process.
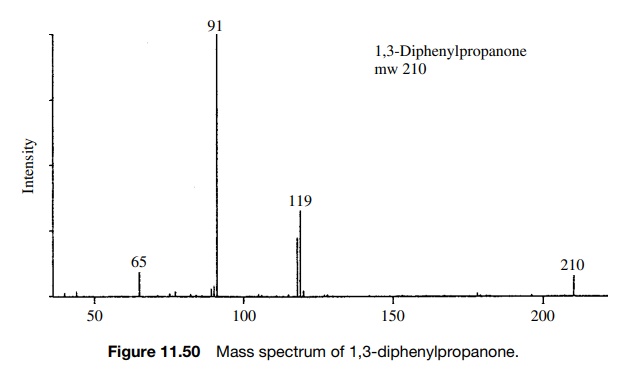
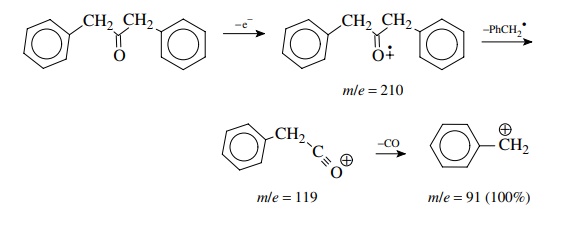
1,3-Diphenylpropanone has a molecular ion at m/e = 210 and significant frag-ment ions of m/e = 119 (M − 15) and m/e = 65. The base peak is m/e = 91. In this example, loss of a benzyl radical from the molecular ion produces an acylium ion (m/e = 119) which rapidly loses CO because the resulting benzyl cation is extremely stable — one of the most stable ions normally encountered. Examination of the mass spectrum (Figure 11.50) shows that there are many additional small peaks present other than those just discussed. Their presence is indicative of the high energy deposited in the molecular ion upon ionization which permits a large number of fragmentations to occur. Nevertheless the frag-mentations which occur most often and lead to the most intense peaks are those that follow common ideas about reactivity and ion stability.
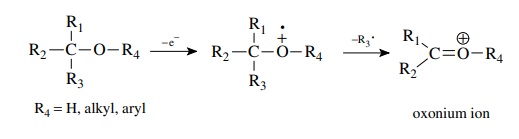
Both
ethers and alcohols readily undergo loss of groups next to the oxygen so as to
produce an oxonium ion. Thus tert-butyl
ethyl ether m/e = 102 has a very large M − 15 peak due to loss of a methyl
radical.
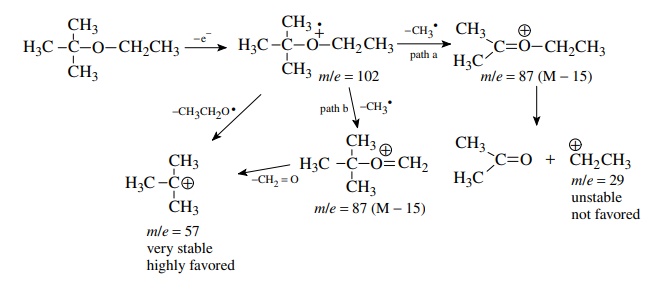
The
methyl group could be lost from either the t
-butyl group (path a) or the ethyl group (path b) to give two different oxonium
ions with the same m/e value. The base peak at m/e = 57 is the t -butyl cation and indicates that at least part of the time the
methyl group is lost from the ethyl group (path b) because subsequent loss of
formaldehyde from the oxonium ion gives the t
-butyl cation. The t -butyl cation
can also be produced by a single fragmentation of the molecular ion by loss of
the ethoxy radical. The stability of the t
-butyl cation makes it the base peak and ensures its production by a variety of
routes. This is not to say that all of the M − 15 peak comes from path b, and most
likely there is some contribution to the m/e
= 87 peak from path
a; however, the oxonium ion thus produced is unlikely to fragment into the very
unstable ethyl cation.
By
working with mass spectral fragmentation patterns, it is possible to develop
very keen insight into the ways that molecules disintegrate under high-energy
conditions. This permits identification of the structure from the pieces and
insight into how they were produced. In conjunction with other structural
tools, MS provides invaluable insight into molecular formula and connectivity
issues in a molecule and is thus an important tool in structure elucidation.
The
foregoing discussion has been a very elementary introduction into MS as a tool
for structure identification. Advances in sample introduction, methods of
ionization, and ion collection and detection have been remarkable, and today
the mass spectra of peptides, nucleic acids, proteins, and other biopolymers
are routinely obtained. Using known cracking patterns, MS is the method of
choice for identifying drugs and drug testing since it requires only minute
quantities (micrograms). It has been sent on the Mars probe to look for amino
acids as an indication of life forms on Mars. One goal of current research
efforts is to use MS as a method for sequencing peptides and oligonucleotides
by their fragmentation patterns. Mass spectrometry is thus an important
analytical and structural tool whose evolution continues at a rapid pace. It
remains an important component of structural investigation.
Related Topics
