General chemistry of group 14 elements
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : The Carbon Group
Occurrence, extraction and use of group 14 elements, Oxidation states and ionisation energies, Typical compounds of group 14 elements
General
chemistry of group 14 elements
Occurrence, extraction and use of group 14 elements
Silicon is, after oxygen, the second most abundant element in
the earth’s crust. It occurs in a range of minerals and sand (SiO2,
quartz). In contrast, germanium, tin and lead are relatively rare elements,
with tin and lead being extracted for thousands of years from their ores. The
main source for tin is cassiterite (SnO2) and for lead galena (PbS).
Germanium was first isolated from the mineral argyrodite when it was
discovered, but there are no major deposits of this mineral. Germanium is
nowadays mainly sourced from zinc and copper ores.
Silicon can be extracted from silicates or
sand by reducing SiO2 with coke at high temperatures at around 3000 ∘C.
SiO2 + 2C → Si + 2CO (5.1)
Germanium is extracted from zinc ores in a very complicated
process as it has aqueous properties simi-lar to those of zinc. Once the
germanium/zinc mixture has been sufficiently enriched with germanium, it is
heated in HCl with Cl2 in order to allow the formation of germanium
tetrachloride (GeCl4). GeCl4 can be easily separated from
ZnCl2 because the former has a significantly lower boiling point,
and after hydrolysis germanium dioxide (GeO2) is obtained. GeO2
can be reduced to elemental Ge in a stream of hydrogen gas. Elemental Sn is
extracted from the ore cassiterite (SnO2) via reduction with carbon.
Lead is obtained from its sulfide (PbS, galena), which is first roasted in the
presence of oxygen and then reduced with carbon to give elemental Pb.
PbS + 11∕2O2 → PbO +
SO2
PbO + C → Pb + CO
Silicon is used in a wide variety of applications. In nature,
silicon does not exist as the pure metal and most commonly occurs in silica
(including sand) and silicates. Silicon dioxide, also known as silica, is a hard substance with a high
melting temperature and clearly very different from carbon dioxide. Molten
silica can be used to make glass, an extremely useful material, which is
resistant to attack by most chemicals except fluorine, hydrofluoric acid and
strong alkalis. Silicon atoms can also be found in the class of compounds
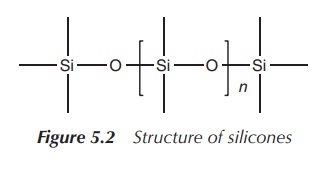
called silicones. Silicones are inert
synthetic polymers with a wide range of uses including as sealants, cookware, adhesives and medical applications.
Silicones contain next to silicon atoms also carbon, hydrogen and oxygen atoms
(Figure 5.2).
Pure silicon metal is used in semiconductors, the basis of all electronic devices, and is most well known for its application in solar panels and computer chips. Germanium can be mainly found not only in electrical components and in semiconductors but also in some optical applications and some specialised alloys.
A semiconductor is a crystalline solid
that has an electric conductivity between that of a conductor (e.g. copper) and
an insulator (e.g. glass). This effect can be a result of impurities or
temperature.
Metallic tin has many applications, but the most well known one
is the use as lining for drink and food cans. It is also used in alloys –
bronze is an alloy of copper and tin – and organotin compounds find their
application in poly(vinyl chloride) (PVC) plastics. Tin is often referred to as
a poor metal and it has two
allotropes: grey tin and white tin.
Lead is a grey metal and most lead is used in batteries. Other
major uses, such as in plumbing or as antiknock agent in petrol (tetraethyl
lead, Pb(C2H5)4), have declined over recent
years because of the high toxicity of lead. Pb is a neurotoxin when ingested
and many lead compounds are water soluble. Therefore, water lines have been
replaced by specialised plastic material, and in most industrialised countries
only unleaded petrol is sold.
Oxidation states and ionisation energies
Group 14 elements can have the oxidation state +2 or +4, with
the latter one being the dominant one. The stability of the oxidation state +2
increases for elements further down the group, such as Sn and Pb. Group 14
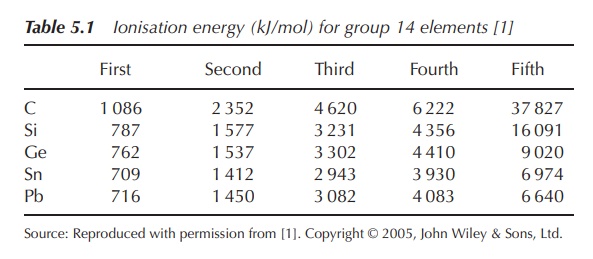
elements have four valence electrons, two in the s orbital and two in the p
orbital. Therefore, the first four ionisation energies increase evenly, whereas
there is a significant increase to the fifth (Table 5.1).
Typical compounds of group 14 elements
The chemistry of group 14 elements is different from that of its
naming element, carbon. The best example is the ability of carbon to form
double and triple bonds. None of the remaining group 14 elements shows the same
behaviour and there are only very few compounds known that contain
silicon–silicon double and triple bonds. Carbon forms double and triple bonds
through the overlap of its 2p orbitals. In contrast, the overlap of the 3p
orbitals in silicon is weaker, and as a result silicon does not form multiple
bonds as readily as carbon.
1. Oxides
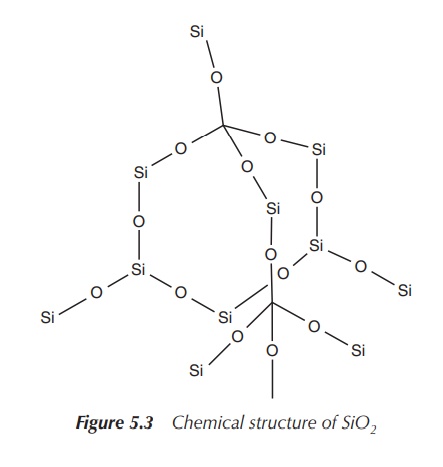
Silicon oxides can be very complex structures, which is in contrast to the simple linear molecule carbon dioxide with its C==O bonds. The most basic molecule is SiO2 (silica), which is an extended array of SiO4 units where the oxygen atoms bridge the neighbouring silicon atoms. Silicon forms an array of oxides that occur in many minerals. All those are made up of SiO4 tetrahedra forming rings or chains with an overall negative charge.
The negative charges are balanced by metal ions
(e.g. alkali and alkaline earth metals). One of the best known examples is the
commercially available synthetic zeolite, which is an aluminosilicate
incorporating aluminium ions. Zeolite is used as an absorbent and in the
production of laundry products (Figure 5.3).
Germanium dioxide has the chemical formula GeO2 and
forms as a passivation layer on pure germanium when the semi-metal is in
contact with atmospheric oxygen. GeO2 forms structures that are
similar to SiO2 depending on the reaction conditions. Germanium monoxide
(GeO) is formed when GeO2 is heated with powdered germanium at 1000∘C. GeO2 is
used in optical devices such as lenses and optical fibres, amongst other
applications.
2. Hydrides
Silicon hydrides are called silanes
and are structurally similar to their carbon analogues, with the general
formula SinH2n+2. Silanes are typically
colourless and can be gases or volatile oils. Germanium hydrides are
accordingly called germanes and these
are generally less reactive than silanes. A classic example is SiH4,
which ignites spontaneously in air whereas GeH4 is stable. Tin
hydrides (SnH4), called stannanes,
are less stable and decompose slowly in air to tin and hydrogen gas. PbH4
has not been isolated or prepared so far, and only hydrides containing organic
substituents have been observed.
3. Halides
Group 14 elements generally form MX4-type
compounds, but germanium, tin and lead also form compounds of the type MX2.
Tetrachlorides of silicon and germanium are important precursors and are used
in the syn-thesis of ultrapure silicon and germanium. These find applications
in the electronic industry as materials for semiconductors. Silicon
tetrachloride instantly hydrolyses when it comes into contact with water.
Related Topics
