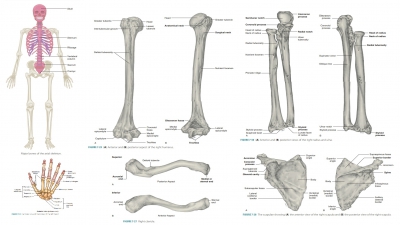
Growth and Development of Bones
| Home | | Anatomy and Physiology | | Anatomy and Physiology Health Education (APHE) |Chapter: Anatomy and Physiology for Health Professionals: Support and Movement: Bone Tissues and the Skeletal System
1. Explain how bones begin to form. 2. Describe the location of the primary and secondary ossification centers in long bones. 3. What is the role of mesenchymal cells? 4. What types of nutrition and hormones influence bone development and growth?
Growth and
Development of Bones
Bones begin to form in utero
during the first eight weeks after fertilization. Intramembranous bones
originate between layers of connective tissues that are sheet-like in
appearance. Examples of intramembranous bones are the flat, broad bones of the
skull. These bones, also called dermal
bones, begin development when unspecialized connective tissues form at the
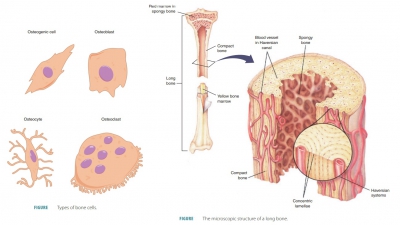
sites where future bones will be developed. Bone-forming cells (osteoblasts)
develop, depositing bony matrix around them. When extracellular matrix has
sur-rounded the osteoblasts, they are termed osteocytes. The surrounding membranous tissues begin to form the
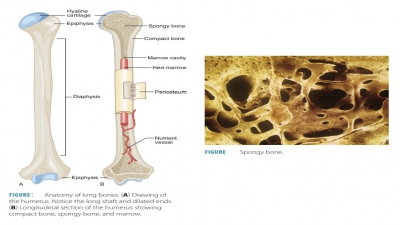
periosteum of a bone. Inside the periosteum, the osteoblasts form a compact
bone layer over the new spongy bone.
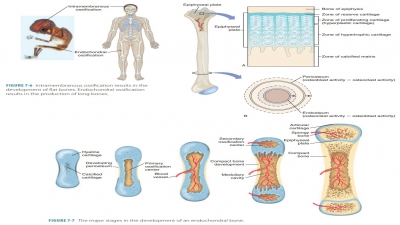
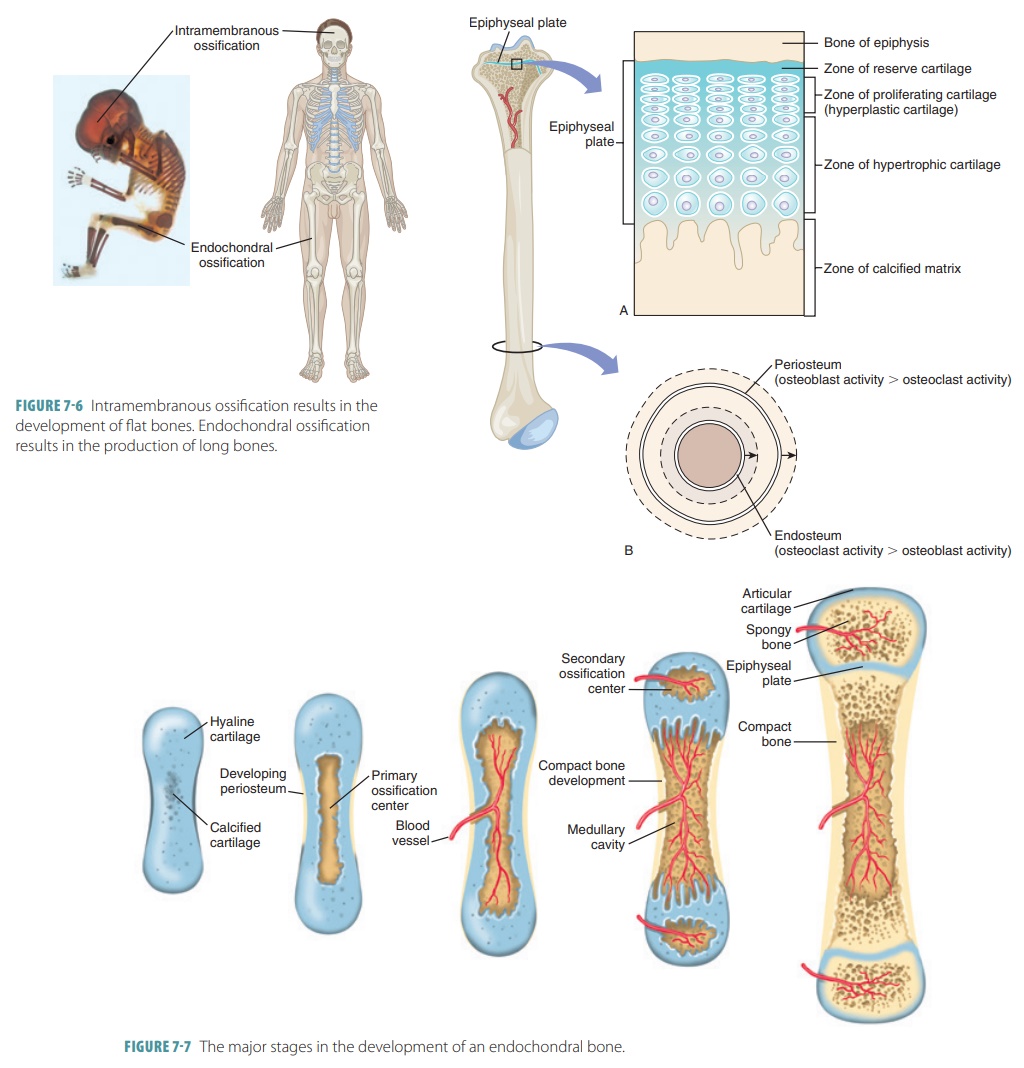
Endochondral bones begin as
cartilaginous masses that are eventually replaced by bone tissue. These bones
develop from hyaline cartilage that is shaped similarly to the bones they will
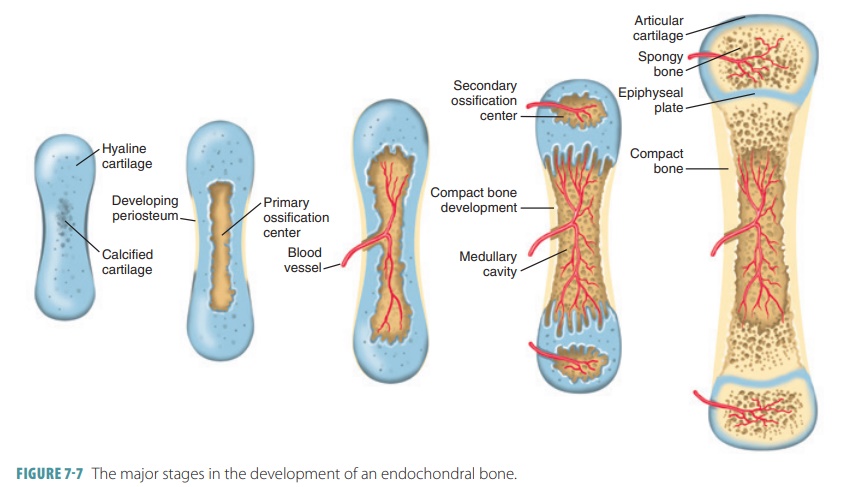
become (FIGURE 7-6). They grow rapidly at first, and then begin to change in appearance.
When spongy bone begins to replace the original cartilage, a primary
ossification center is created, with bone tissue developing outward toward the
ends of the structure. Eventually, secondary ossifi-cation centers appear in
the epiphyses, forming more spongy bone.
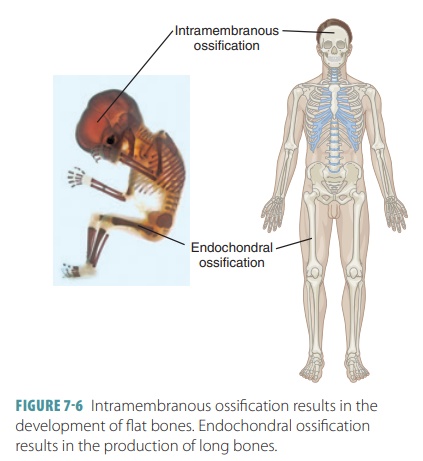
During the first eight weeks of
development, the skeleton is cartilaginous. The bones increase greatly in size
as the fetus develops and throughout childhood (FIGURE
7-7). Bone growth continues through
adoles-cence. The process of replacing other tissues with bone is called ossification, which
involves the deposition of calcium salts. In embryos, ossification (as well as
osteogenesis) leads to formation of the skeleton. Later in development, bone growth occurs until early
adult-hood. Throughout life, the bones can become thicker. Osteogenesis is defined as the formation of bone.
The skeleton of a human embryo,
before week eight of gestation, is made of fibrous membranes and hyaline
cartilage. At week 8, bone tissue begins to develop, replacing most existing
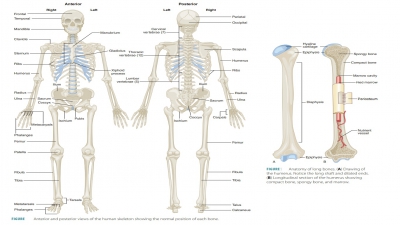
cartilage or fibrous structures over time. For example, the long bones are
first formed of hyaline cartilage and later replaced by bony tissue that
becomes compact bone. This process begins with the diaphysis and ends with the
epiphyses of each long bone. This process of bone formation is called endochondral ossification. Cartilage is also referred to as endochondral
bone.
Nearly all bones below the base
of the skull (except the clavicles) are formed by endochondral ossification. At
month two of gestation, hyaline cartilages previously formed are used as models
for actual bones. Endochon-dral ossification is more complicated than
intramem-branous ossification, because hyaline cartilage is broken down while
ossification is occurring. For long bones, the center of a hyaline cartilage
shaft (the primary ossification center) is where
ossification begins. Blood vessels form in the perichondrium that covers the
hyaline cartilage bone model and convert it to a vascu-larized periosteum. As
nutrients become more plenti-ful, underlying mesenchymal cells specialize to
become osteoblasts. This becomes the basis for ossification.
Bone collars form around the
diaphysis of the hyaline cartilage models, as osteoblasts secrete osteoid
against the hyaline cartilage diaphysis. They enclose it in a collar-like
structure known as the periosteal bone
collar. In the center of the
diaphysis, the cartilage cal-cifies, developing cavities. Chondrocytes in the
shaft enlarge (hypertrophy), which leads to the surrounding cartilage matrix to
calcify. Because this matrix cannot be penetrated by diffusing nutrients,
chondrocytes die. The matrix deteriorates, opening up cavities. The bone collar
stabilizes the hyaline cartilage model. In other locations, the cartilage is
still healthy and grow-ing quickly, which lengthens the cartilage model.
By the third month, the cavities
are invaded by elements, collectively known as the periosteal
bud. This contains a nutrient artery
and vein, red marrow elements, nerve fibers, osteoclasts, and osteogenic cells.
The calcified cartilage matrix is partially eroded by the osteoclasts. The
osteogenic cells become osteo-blasts, secreting osteoid around calcified fragment
of hyaline cartilage. This forms a bone-covered cartilage trabeculae.
Therefore, an early version of spongy bone develops in the long bone.
Osteoclasts break down the new
spongy bone as the primary
ossification center enlarges. A med-ullary cavity is
opened in the center of the diaphy-sis. From week nine until birth, the quickly
growing epiphyses are made only of cartilage. The hyaline car-tilage models
continue lengthening via the division of viable cartilage cells at the
epiphyses. Down the length of the shaft, cartilage calcifies, erodes, and is
replaced by spiked bone structures on epiphyseal surfaces that face the
medullary cavity.
At birth, most long bones have a
bony diaphysis that surrounds spongy bone remnants, a medullary cavity that is
widening, and two epiphyses made of cartilage. Secondary ossification centers develop in one or both epiphyses just before or just after birth.
Usually, secondary centers form in both epiphyses of larger long bones, and in
smaller long bones, usually only one secondary ossification center forms. The
cen-tral cartilage of the epiphysis calcifies and deteriorates. Cavities open,
allowing a periosteal bud to enter. Bone trabeculae appear similar to how they
appeared in the primary ossification center. Short bones develop dif-ferently
in that only the primary ossification center is formed, and most irregular
bones have several distinct ossification centers from which they develop.
Secondary ossification is very
similar to primary ossification, except for interior spongy bone being retained
and the lack of a medullary cavity being formed in the epiphyses. Hyaline
cartilage remains only on the epiphyseal surfaces (as articular cartilages) and
at the junction of diaphyses and epiphyses (forming epiphy-seal plates) when
secondary ossification is complete.
Flat bones are not formed in the
same way as long bones. Flat bones develop from fibrous connective tissue
membranes (formed by mesenchymal
cells) that are replaced by spongy
bone, and then compact bone in a process is called intramembranous ossifica-tion. The produced bones are also referred
to as mem-brane bones. In the
embryonic skeleton, membranes and
cartilages allow for mitosis. Examples of flat bones formed via intramembranous
ossification include the frontal, parietal, occipital, and temporal bones of the
skull and the clavicles. Ossification begins at about week eight of gestation.
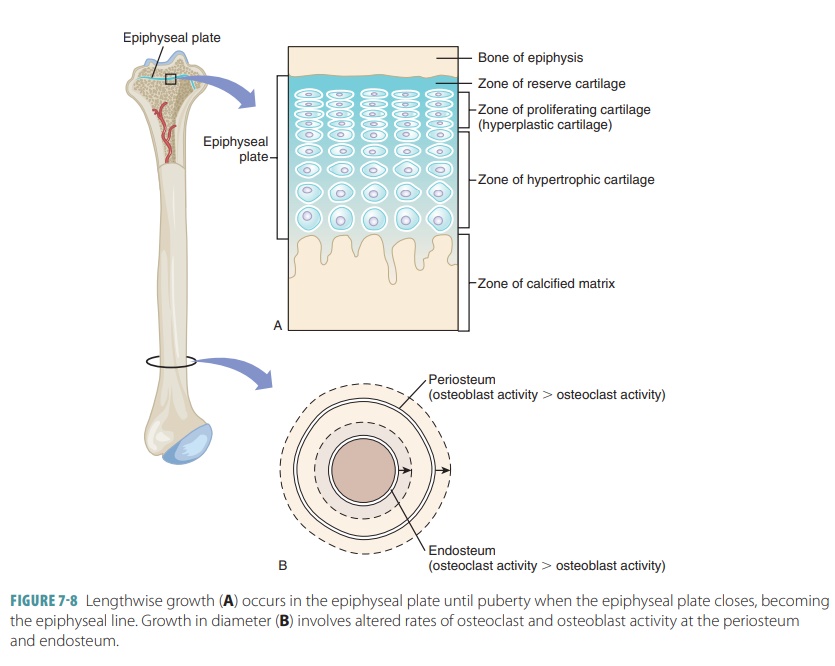
When the bones are growing, the
diaphyses meet the epiphyses at a structure called the epiphyseal plate. It is made of four cartilage layers: reserve
cartilage, prolifer-ating (hyperplastic) cartilage, hypertrophic cartilage, and
the calcified matrix. Growth of long bones depends on good nutrition and
several hormones, including human growth hormone. Interstitial growth of the
epiphyseal plate cartilage and then replacement by bone is responsi-ble for all
long bone growth after birth. All bones grow in thickness by appositional
growth. The other hormones involved in long bone growth include thyroid
hormone, estrogen, and testosterone. Once the epiphyseal plate experiences
closure, the long bones can no longer grow (FIGURE
7-8). Length of bone is balanced by
increased bone width. Osteoblast and osteoclast activity is balanced in the
body, so bones grow with uniformity. Most bone growth stops during adolescence,
although the bones of the nose and lower jaw (as well as other facial bones)
may continue to grow in only tiny increments throughout life.
Bone development, growth, and
repair are influenced by nutrition, hormones, and exercise. Vitamin D is
required for the absorption of calcium in the small intestine. Without it,
calcium is not absorbed well, softening bones and potentially causing
defor-mity. Growth hormone from the anterior pituitary gland stimulates cell
division in the epiphyseal plates. During infancy and childhood, growth hormone from the anterior
pituitary gland determines epiphyseal plate growth activity. This hormone is
modulated by thyroid hormones, so the skeleton develops the proper proportions
during growth. Calcitriol,
syn-thesized from another steroid called cholecalciferol
(vitamin D3), is made by the kidneys. It is essential for normal
phosphate and calcium ion absorption in the digestive tract. Cholecalciferol is
produced in the skin or absorbed from the diet. Vitamin C must also be present
in the diet, because it is needed for import-ant enzyme reactions in collagen
synthesis and to stimulate osteoblast differentiation. Vitamin A stimulates
osteoblast activity and vitamins C, K, and B12 are essential for
synthesis of normal bone proteins.
At puberty, male and female sex
hormones (testosterone and estrogen, respectively) stimulate ossification of
these plates. Thyroid hormone modulates the activity of growth hormone. Exercise
stresses the bones, stimulating them to become these hormones. If growth
hormone is excessively secreted, gigantism occurs. Similarly, hormone deficits
result in various forms of dwarfism.
1. Explain
how bones begin to form.
2. Describe
the location of the primary and secondary ossification centers in long bones.
3. What is
the role of mesenchymal cells?
4. What
types of nutrition and hormones influence bone development and growth?
Related Topics