History and Organisation
| Home | | Pharmacovigilance |Chapter: Pharmacovigilance: Spontaneous Reporting - France
To understand the way it functions, and some of the differences with other countries’ pharmacovigilance systems, a little history is necessary.
HISTORY AND ORGANISATION
To
understand the way it functions, and some of the differences with other
countries’ pharmacovigilance systems, a little history is necessary. After the
thalidomide tragedy, and the other early drug safety scandals or scares, a
number of clinical toxicologists and pharmacologists, usually associated with
Poison Control Centres (Paris, Lyon, Marseille), decided to set up units to
inform their physicians of the risks of drugs, and provide for a local place to
report ADRs. In 1973, a national centre was set up by the French Medical
Association in collaboration with the French Pharmaceutical Manufacturers
Associa-tion. The same year, six experimental pharmacovigi-lance centres were
created in France. Over the years, more pharmacologists joined the first ones,
and a network of centres appeared. The heads of these centres, at the time
without any official remit, met regularly during meetings of the French
Associa-tion of Pharmacologists. As this network evolved, they had to work out
common methodologies. From the mid-1970s the centres were officially
recog-nised, the regular meetings started taking place at the Ministry of
Health, and a unit was set up there to co-ordinate activities. In 1979 a
decentralised system was put in place with a network of 15 centres, which was
thereafter extended to 29 in 1984 and 31 in 1994. Since 1984, prescribers
(physicians, dental surgeons and midwives) and marketing authorization holders
(MAHs) have been required to report ADRs. The national database was rejuvenated
in 1985 so that online input became possible, and it could be accessed from all
centres. In 1994 the Pharmacovig-ilance Unit was transferred to the French
Medicines Agency (now French Agency for the Safety of Health Products,
AFSSAPS). Good Pharmacovigilance Prac-tices were evolved and sent to every
prescriber in the country. To implement the European legisla-tion, two decrees
came into force which extended the mandatory reporting of ADRs to pharmacists
and defined the current general organisation of the French pharmacovigilance
system: the decree of March 1995 on general principles and the decree of May
1995 that especially related to human blood products.
At
the present time, the 31 Regional Centres have a duty to collect, record and
evaluate ADR reports, and input them into the common database, after causal-ity
assessment. The Heads of the Regional Pharma-covigilance Centres meet monthly
at the AFSSAPS in the Technical Committee, a working group set up to prepare
the work of the National Pharmacovig-ilance Commission (Advisory Board). The
Techni-cal Committee is responsible for co-ordinating the collection and
evaluation of information on ADRs, conducting surveys and providing
recommendations that are forwarded to the National Pharmacovigi-lance
Commission, which recommends action to the General Director of the Agency, to
prevent or elimi-nate drug-related accidents (Figure 16.1).
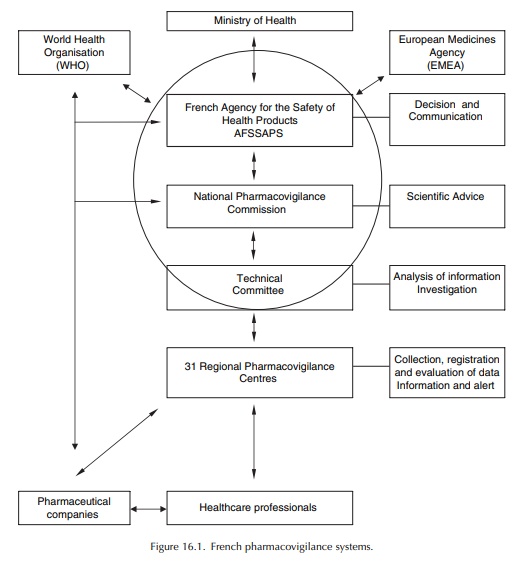
The AFSSAPS is responsible for implementing the national pharmacovigilance system. It defines the pharmacovigilance trends and co-ordinates the actions of the various partners involved. The Phar-macovigilance Unit of AFSSAPS centralises all the data collected on the territory by the regional phar-macovigilance centres (via the national database) and the pharmaceutical companies (who report directly ADRs to the Unit). This Unit is in charge of the co-ordination of the Regional Centres’ activities, the organisation of meetings held by the Technical Committee and the National Pharmacovigilance Commission, and the exchange of information with other competent authorities: the European Medicines Agency (EMEA), other Member States, the World Health Organisation (WHO), competent authorities in third countries (Food and Drug Administration (FDA), etc.). It also monitors compliance with pharmacovigilance regulatory obligations of each partner involved, especially to ensure that the report-ing requirements are fulfilled. The AFSSAPS takes appropriate measures to ensure the safe use of medic-inal products after marketing with the same objective: to protect public health.
Related Topics
