Arsenic
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : Group 15 Elements
Arsenic is a metalloid of the nitrogen group. Two allotrope forms of elemental arsenic have been reported: yellow arsenic and grey arsenic, the latter being usually the more stable form.
Arsenic
Arsenic is a metalloid of the nitrogen group. Two allotrope
forms of elemental arsenic have been reported: yellow arsenic and grey arsenic,
the latter being usually the more stable form. Arsenic readily oxidises in air
to arsenic trioxide (As2O3). Arsenic is mostly found
either in its native state or as arsenic sulfide in the form of realgar (As4S4)
or orpiment (As2S3). Arsenic can exist in three different
valence states (zerovalent, trivalent and pentavalent). Arsenic forms covalent
bonds with carbon, oxygen and hydrogen. The toxicity varies widely and depends
on the physical state of the compound and its absorption/elimination rate.
Trivalent arsenics (As(III)) are derivatives of the arsenous acid (H2AsO3 – arsenite) and arsenic trioxide (AsO3). Examples of pentavalent arsenic (As(V)) include derivatives of the arsenic acid (H3AsO4 – arsenate).
Organic arsenic-based compounds, that is, compounds containing
arsenic–carbon bonds, are usually less toxic than their inorganic counterparts.
This is mainly due to their quicker excretion from the human body.
Arsenic is known to be one of the most toxic heavy metals.
Compounds containing arsenic have a long history of use as poisons, but they
also have a long historical medicinal use. As2O3, As2S3
(orpiment) and As2S2 (realgar) have been used as early as
2000 BC as drugs, for example, to cure cancerous tumours, ulcers and other
diseases of the time. Nevertheless, the therapeutic use of arsenic-based compounds
con-tinued; for example, Galen (130–200 AD) recommended the application of a
paste of arsenic sulfide against ulcers. Paracelsus ‘ignored’ any kind of
formulation and recommended the clinical use of elemental arsenic. Fowler’s
solution (1% potassium arsenite) was applied in a variety of clinical
applications. Interestingly, it was the main treatment option for chronic
myelogenous leukaemia (CML) until it was replaced by radiation and chemotherapy
in the twentieth century. Again, until the twentieth century, arsenic-based
drugs were, for example, mostly used to combat trypanosomal infections. Indeed,
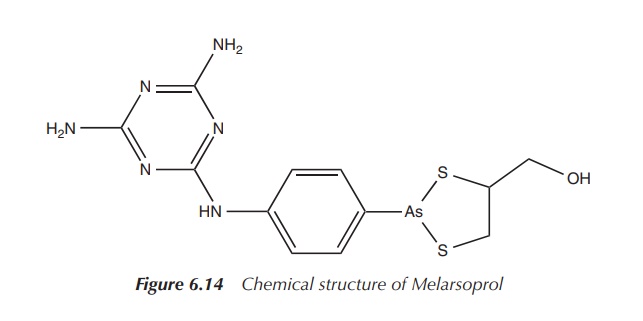
Melarsoprol is currently approved for the treatment of late-stage African
trypanosomiasis (Figure 6.14).
Salvarsan: the magic bullet – the start of chemotherapy
Salvarsan was a synthetic arsenic-based drug discovered in 1909
by Ehrlich and his team. In 1910, Ehrlich introduced Salvarsan
(3-amino-4-hydroxyphenylarsenic), also known as arsphenamine or compound 606,
to the market as a cure for syphilis caused by the bacterium Treponema pallidum.
1. Historical developments
Early in his studies, Ehrlich believed in the search for the
‘magic bullet’ – a treatment that would result in ‘the use of drugs to injure
an invading organism without injury to the host’ . This can be regarded as the
start of chemotherapy. Early in his research career, Ehrlich became interested
in bacteriology and the use of aniline and other dyes to selectively stain
bacteria. In 1904, Ehrlich used trypan red to selectively stain trypanosomas
(protozoa responsible for the African sleeping sickness). He discovered that no
other cells took up the dye, and he got the idea of selectively targeting
single cells from this experiment – the early start to chemotherapy. Ehrlich
and his team managed to show that mice infected with trypanosomas could be
cured with trypan red, but human experiments failed. Even today, trypan blue as
staining agent is used to distinguish between living and dead cells because
living cells do not take up the dye .
In 1905, the bacterium T. pallidum was identified by Schaudinn and Hoffmann as the cause syphilis. This discovery inspired Ehrlich to search for a cure using his targeted approach. ‘We must search for magic bullets’ , Ehrlich commented during his research. ‘We must strike the parasites and the parasites only, if possible, and to do this, we must learn to aim with chemical substances’ .
Béchamp, teaching medicinal chemistry at the University of
Montpellier, synthesised in 1863 a compound from aniline and arsenic acid,
which became known later as Atoxyl.
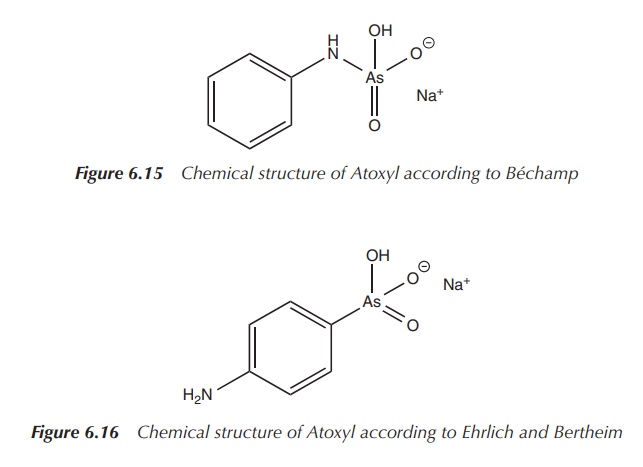
The name Atoxyl stems from its decreased toxicity. Béchamp characterised his
compound as an anilide, and its structure is shown in Figure 6.15 .
In 1905, Thomas and Breinl showed that Atoxyl was effective in
the treatment of trypanosomas, mainly Trypanosoma
brucei gambiense – the cause of the African sleeping sickness, which was
the main health problem around that
time in Africa. Nevertheless, very high doses were required to show any
pronounced effect, and as a result severe side effects such as blindness and
damage to the optical nerve were common issues .
Inspired by this research, Ehrlich hired the chemist Bertheim in 1905. Bertheim revised the structure of Atoxyl and the correct chemical formula was assigned. Atoxyl was identified as an p-anilinyl arsenic acid derivative on the basis of its properties to reduce Tollen’s reagent [Ag(NH3)2]+ to metallic silver and its poten-tial use to synthesise the corresponding diazo dye. Diazotisation is possible only for primary aromatic amines and therefore it could be concluded that Atoxyl had to be an arsenic acid rather than an anilide derivative; the correct structure according to Ehrlich and Bertheim is shown in Figure 6.16 .
Ehrlich’s coworker, Hata, discovered a way to infect rabbits
with T. pallidum. No one before had
been able to produce syphilis in an animal, and in 1909 the first successful in vivo experiments in rabbits were
performed. Having identified the correct structure of Atoxyl, Ehrlich and his
team were inspired to search for a huge number of derivatives. Eventually,
compound 606 was synthesised and introduced as an agent against syphilis. The
compound was later marketed as Salvarsan, receiving its name from the Latin
word ‘salvare’, which means to preserve, to heal. In 1909 and 1910, the first
human tests on patients with syphilis and relapsing fever were extremely
successful, and Salvarsan was marketed from 1910. For the first time, an
infectious and fatal disease in humans could be treated with a man-made
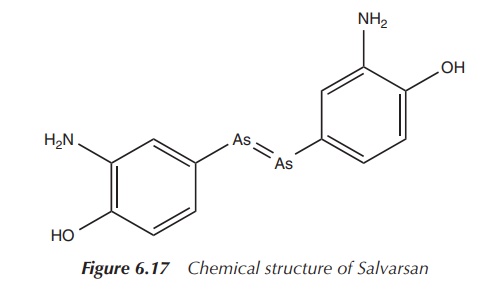
molecule, and Salvarsan brought Ehrlich world-wide fame (Figure 6.17) .
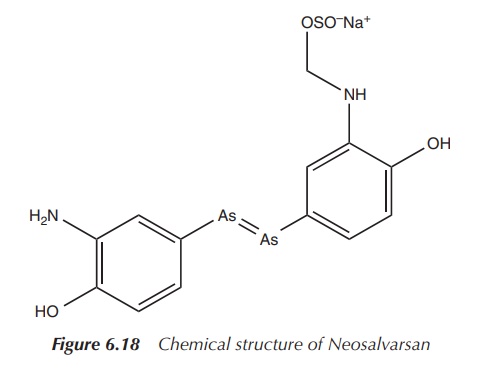
However, Ehrlich and his team did not stop with the discovery of Salvarsan. In particular, formulation issues encouraged them to search for a derivative which was easier to administer in order to make an injectable solution. Neosalvarsan (compound 914) is a salt derivative of Salvarsan and is water soluble, which showed reduced side effects (Figure 6.18) .
Around a decade later, doubts arose about the stability of an As==As double bond, as analysis of the arsenic
content of the samples never conformed to the structure stated. Later work
showed that neither Salvarsan nor Neosalvarsan was the active pharmaceutical
ingredient (API). In 1930, the oxidised compound Oxophenar-sine, containing an
As==O unit, was identified as the active
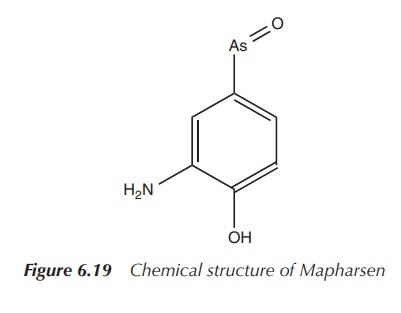
ingredient and was later marketed under the trade name Mapharsen. Mapharsen was
used until the 1940s when it was replaced by Penicillin. Mapharsen was actually
synthesised in Ehrlich’s laboratory as compound number 5, but it was believed
to be too toxic for any clinical application (Figure 6.19) .
Generally, the use of arsenic-based drugs has ceased, especially
as a result of the development of Penicillin. Nevertheless, Melarsoprol and an
arsenic-based drug closely related to Atoxyl are licensed to treat sleeping
sickness.
2. Synthesis and structural analysis of Salvarsan
In Ehrlich’s time, it was very reasonable to
formulate the structure of Salvarsan as he did. But the As==As double bonds are not stable under the
reaction conditions chosen by Bertheim and Ehrlich. Their proposed synthetic
route was based on the reaction of 3-nitro-4-hydroxyphenyl-arsonic acid with
dithionite.
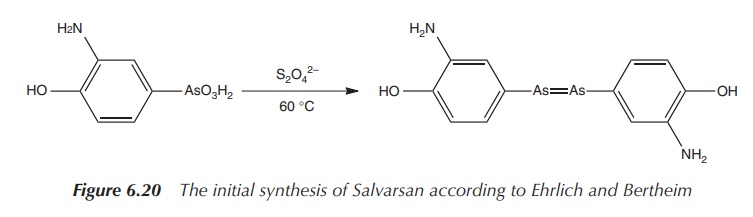
As a result, the nitro group is reduced to an amine group and simultaneously
As(V) is reduced to As(I), resulting in a compound with the formula 3-H2N-4-HOC6H3As
(see Figure 6.20). The product was then isolated as the hydrochloride salt 3-H2N-4-HOC6H3As⋅HCl⋅H2O. Unfortunately, this synthetic route was not
always repro-ducible .
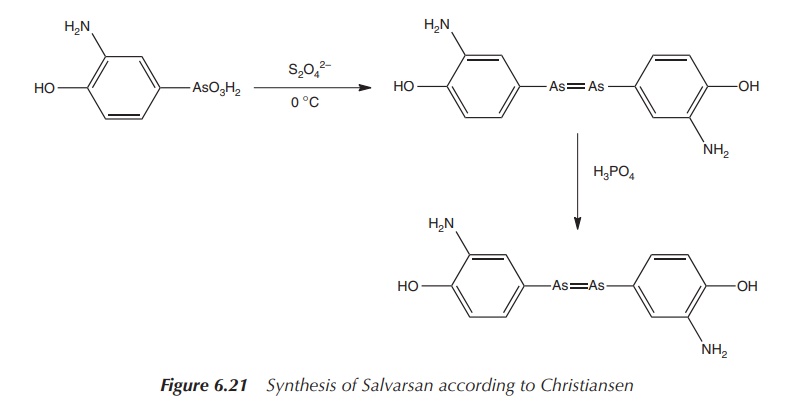
Christiansen et al.
published in 1920 a two-step process leading to the sulfur-free product. The
reaction involved the initial reduction of the nitro group with sodium
dithionite and the subsequent reduction of the As(V) with hypophosphorous acid
(Figure 6.21) .
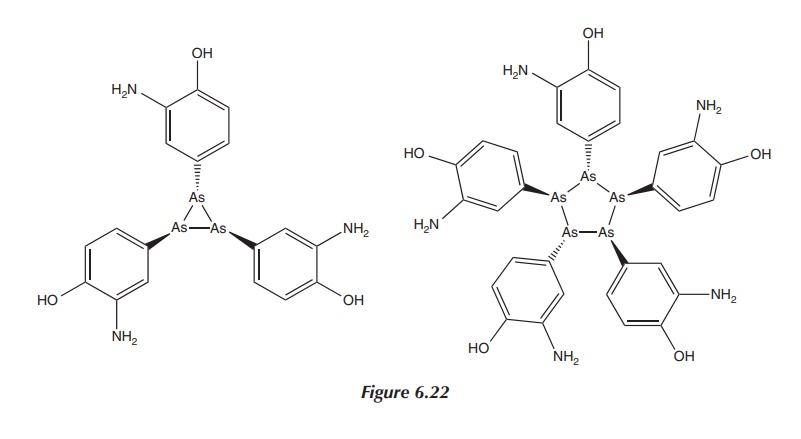
Nevertheless, subsequent research has shown that dimeric
arsenic-based structures exist only in sterically crowded molecules. The real
structure of Salvarsan is not dimeric. Research published in 2005 by Lloyd et al. using different mass
spectroscopic techniques showed that Salvarsan in solution consisted of small
cyclic species with ring sizes of three and five arsenic atoms (see Figure
6.22). Nevertheless, this final structure of Salvarsan has still not been
entirely identified .
Arsenic trioxide: a modern anticancer drug?
Arsenic trioxide, often denoted as As2O3
but more correctly stated as As4O6, is an inorganic
compound mainly used as the precursor for organoarsenic compounds. It can be
obtained by the oxidation of arsenic-containing minerals in the air, such as
roasting of orpiment.
2As2S3 + 9O2 → As4O6 + 6SO2
As4O6 is sparingly soluble in water and is
an amphoteric compound. It reacts with alkali with the formation of arsenates,
and arsenite trichlorides are synthesised in the presence of an acid.
As4O6 + 12NaO → 4Na3AsO3
+ 6H2O
As4O6 + 12HCl → 4AsCl3
+ 6H2O
Arsenic trioxide is highly toxic. It is
readily absorbed in the digestive system, through inhalation and skin contact.
With a half-life of 1–2 days, elimination occurs rapidly at first via a
methylation reaction and excre-tion in the urine. Around 30–40% of arsenic
trioxide is incorporated into bones, muscles, hair and nails.
This means that elimination can take months, and any arsenic
poisoning is detectable for the same period. Arsenic poisoning is characterised
by digestive problems such as vomiting, diarrhoea and abdominal pain, as well
as cardiovascular problems. Lower doses can lead to liver and kidney damage as
well as changes in the pigmentation of the skin and nails (occurrence of
so-called Mees stripes) .
Nevertheless, arsenic trioxide is long known for its therapeutic
properties especially in the traditional Chi-nese medicine and homeopathy. In
the latter, it is known as arsenicum
album (dilution of arsenic trioxide). Despite its toxicity, arsenic
trioxide and its derivatives have found application in the treatment of cancer.
In 1878, Fowler’s solution (1% potassium arsenite) showed a reduction of white
blood cells when administered to healthy people and patients with leukaemia. It
was reported in 1930 that arsenic trioxide was effective in patients with CML.
It was used after radiation therapy until modern chemotherapy replaced this
treatment approach .
Arsenic trioxide – marketed under the trade name Trisenox –
gained FDA approval in 2000 for the treat-ment of acute promyelocytic leukaemia
(APL). Trisenox, an injectable formulation, has been licensed for use in
patients with induction of remission with APL after all-trans retinoic acid (ATRA) and anthracycline chemotherapy, and
where APL is characterised by the presence of the t(15;17) translocation or
PML/RAR-α (promyelocytic leukemia/retinoic acid receptor-alpha) gene
expression. Trisenox was also approved in 2002 by the European Agency for the
Evaluation of Medical Products for the treatment of adults with relapsed APL .
APL is a subtype of acute myelogenous
leukaemia (AML), which is a cancer of the blood and bone marrow. The disease is
caused by a chromosomal translocation involving the RAR-α gene and therefore
unique com-pared to other forms of AML in its response to ATRA therapy.
Unfortunately, about 20–30% of patients do not achieve remission from the
combination of ATRA and cytotoxic chemotherapy, or they relapse. Trisenox was
reported to achieve a 70% complete response rate in patients with APL which
relapsed after treatment with cytotoxic chemotherapy and ATRA .
Related Topics
