Ruthenium
| Home | | Inorganic Pharmaceutical Chemistry |Chapter: Essentials of Inorganic Chemistry : Transition Metals and d-Block Meta Chemistry
Ruthenium is the chemical element with the symbol Ru and atomic number 44. It occurs as a minor side product in the mining of platinum. Ruthenium is relatively inert to most chemicals. Its main applications are in the area of specialised electrical parts.
Iron and ruthenium
Group 8 of the periodic table of elements consists of the nonradioactive members iron (Fe), ruthenium (Ru) and osmium (Os), as well as the radioactive element Hassium (Hs) (Figure 7.40).
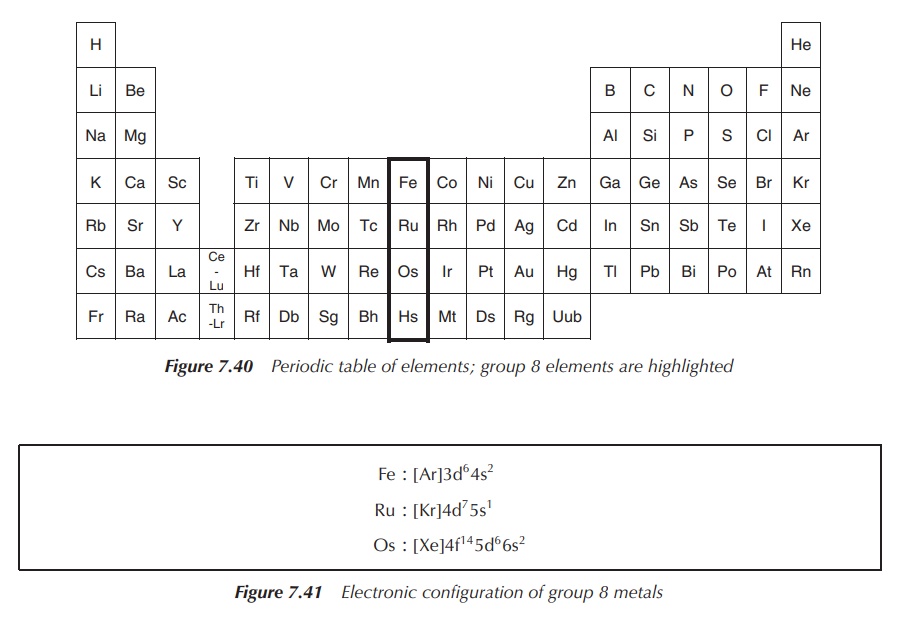
All three nonradioactive elements are silvery white, hard transition metals with a high melting point. Iron has been classified as the most common element within the entire earth, as most of the earth’s core is iron. In contrast, ruthenium and osmium are two of the rarest elements on earth. The radioactive element hassium has not been isolated in pure form yet and therefore its exact properties have not been established. It has only been produced in nuclear reactors and never has been isolated.
The electronic configuration of group 8 metals is shown in Figure 7.41.
Ruthenium
Ruthenium is the chemical element with the symbol Ru and atomic
number 44. It occurs as a minor side product in the mining of platinum.
Ruthenium is relatively inert to most chemicals. Its main applications are in
the area of specialised electrical parts.
The success of cisplatin, together with the occurrence of
dose-limiting resistances and severe side effects such as nausea and
nephrotoxicity, encouraged the research into other metal-based anticancer
agents. Ruthe-nium is one of those metals under intense research, and first
results look very promising, with two candi-dates – NAMI-A and KP1019 – having
entered clinical trials.
1. Ruthenium properties and its biology
Ruthenium has mainly four properties that make it an interesting
candidate for drug development: (i) the range of oxidation states, (ii) low
toxicity compared to cisplatin, (iii) rate of ligand exchange and (iv) ability
to mimic iron binding in biological targets.
Under physiological conditions, ruthenium can be found in
several oxidation states such as II, III and IV, and the energy barrier for
conversion between these oxidation states is fairly low. Ru(II) and Ru(III)
have been extensively used in drug design, and they preferentially form
six-coordinated octahedral complexes. Ru(III) species are the most inert
biological species compared to the Ru(II) and Ru(IV) compounds. The redox
poten-tial of a complex depends on the ligands and can therefore be modified.
For pharmaceutical applications, a Ru(III) complex can be so engineered that it
easily reduces to the Ru(II) compound. In a biological environ-ment, Ru(III)
and Ru(IV) can be reduced by biomolecules such as GSH and ascorbate. In turn,
Ru(II) species can be oxidised by molecular oxygen or enzymes such as
cytochrome oxidase. Cancer cells are known to have a generally reducing
environment. Therefore, ruthenium complexes can be administered as inert
Ru(III) com-pounds, which are then in
situ reduced to the active Ru(II) species . This would mean that minimal
damage is caused to healthy cells whilst cancerous cells become the target of
the active ruthenium species; the result is that ruthenium compounds show a
significantly lower toxicity than platinum complexes. Nevertheless, this
theory, called activation by reduction
has been questioned in recent years.
Ligand exchange is an important factor for the activity of
metal-based drugs, especially anticancer agents. Only very few metal drugs
reach their target in the form they have been administered. Most undergo rapid
ligand exchange as soon as there are interactions with macromolecules or water.
Some interactions are crucial for the activation of metal-based drugs, but
often they hinder their activity. Ruthenium complexes have similar ligand
exchange kinetics as cisplatin, with Ru(III) compounds being the most inert
complexes. In contrast to cisplatin, which forms square planar complexes,
ruthenium complexes are octahedral and therefore there is room for two more
ligands compared to cisplatin to engineer the potential drug.
Iron and ruthenium are both members of group 8 within the
periodic table of elements, and therefore researchers have inquired whether
ruthenium can utilise the transport mechanisms normally used by iron. Iron is a
key element for many biological processes; nevertheless, it is toxic for
biological systems in its isolated form. It is believed that the low toxicity
of ruthenium is a result of its iron-mimicking ability. There have been several
hypotheses suggesting that ruthenium could use transferrin and albumin as
transport mechanisms instead of iron or in a ‘piggy-back’ mechanism where
ruthenium binds to the outside of transferrin when it is loaded with iron.
2. Ruthenium-based anticancer agents
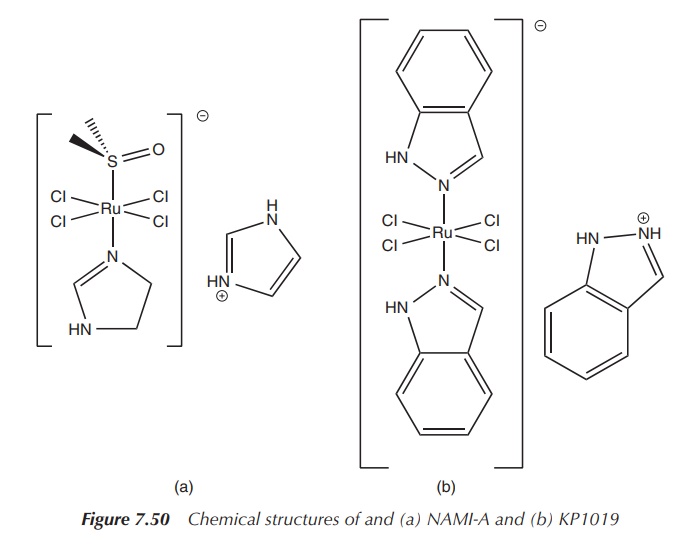
KP1019 and NAMI-A are two ruthenium compounds that have entered clinical trials. They are classical coordination compounds in which the central ruthenium atom is coordinated by Lewis bases in an octahedral arrangement.
Both complexes are based on a Ru(III) core, and the
ligands are typically chloride and organic groups. This means that the complex
can easily be hydrolysed and converted into its active form. Despite their
similar structures, both ruthenium compounds present a different anticancer
activity. KP1019 is active against primary cancers, whereas NAMI-A is active
against metastasis, secondary tumours that have moved to other areas. The
treatment of metastasis is currently an area where improvement is urgently
needed (Figure 7.50).
NAMI-A has shown activity in the treatment of metastatic cancer
and has completed phase I clinical trials in the Netherlands. It has been shown
that the complex is relatively nontoxic. Significantly higher doses than
cisplatin (above 500 mg/m2/day) lead to side effects such as
blisters on the extremities. Within the study, the ruthenium complex was
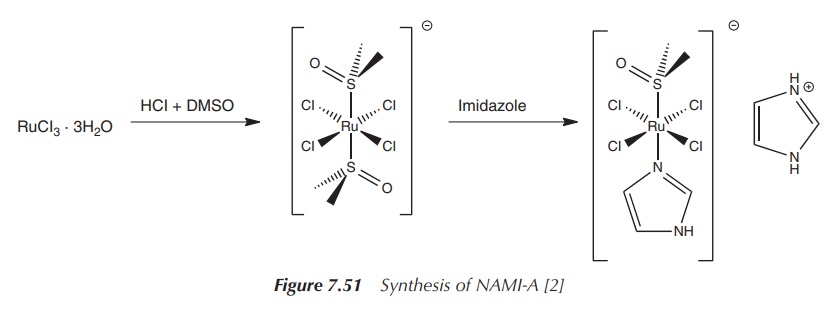
administered intravenously over a period of 3 h in a 0.9% saline solution (pH ∼ 4) . The NAMI-A is synthesised by reacting
RuCl3⋅3H20
with HCl and DMSO (dimethylsulfoxide). This reac-tion results in the trans complex Imidazolium trans-imidazoledimethyl
sulfoxide-tetrachlororuthenate(III) (NAMI-A)
(Figure 7.51).
It is interesting to note that Imidazolium trans-imidazoledimethyl sulfoxide-tetrachlororuthenate(III) is a
paramagnetic compound and the complex is quickly hydrolysed in water.
Initially, one chloride ligand is replaced by an aquo ligand, but the DMSO
ligand is quickly replaced as well. As previously mentioned, it has been
suggested that the complex is activated by bio-reduction of the Ru(III) centre
to Ru(II) in the hypoxic environment of cancer cells. There is not much
knowledge at present about the biological target for Imidazolium trans-imidazoledimethyl
sulfoxide-tetrachlororuthenate(III). It is known that it interacts with the
imidazoles of proteins and that the interaction with DNA is only weak,
questioning DNA as a primary target for the ruthenium drug.
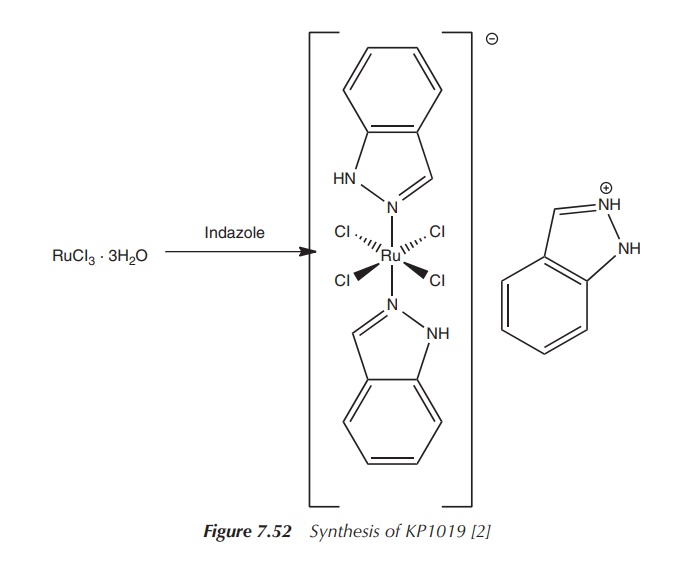
KP1019, trans-[tetrachloro-bis(1H-indazole)ruthenate(III)], was tested
in phase I clinical trials as a pos-sible treatment option against colon
cancer. Administered doses ranged from 25 to 600 mg twice weekly, and no
significant side effects were noticed. The synthesis of KP1019 also starts with
RuCl3⋅3H20,
which is dissolved in ethanolic HCl and reacted with an excess of indazole
(Figure 7.52).
The ruthenium complex hydrolyses quickly in water and induces
apoptosis in cancer cells, potentially by blocking the mitochondrial function.
KP1019 is believed to be transported by the protein transferrin to the tumour
cells which are known to have a greater number of transferrin receptor on their
cell surface.
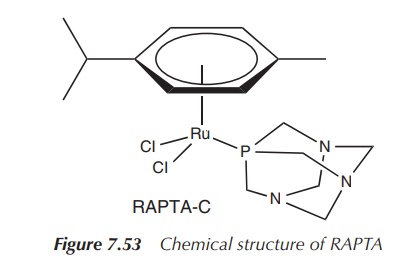
RAPTA is an organometallic ruthenium complex
(see Chapter 8 for the definition of organometallic complex) which is highly
water-soluble. The Ru(II) centre is coordinated by chloride, an aromatic ring
and 1,3,5-triaza-7-phosphatricyclo-[3.3.1.1]decane which is a form of phosphaadamantane.
Interestingly, it shows similar biological activity as Imidazolium trans-imidazoledimethyl
sulfoxide-tetrachlororuthenate(III) despite the differences in geometry,
ligands and oxidation state. This shows that the active species in the cancer
cell is different from the administered active pharmaceutical ingredient (API)
(Figure 7.53).
In summary, the mode of action for ruthenium complexes is still
under investigation, and there are a variety of biological targets. It is
believed that interaction with proteins significantly contributes to their
anticancer activity. Nevertheless, a number of ruthenium complexes have been
shown to bind to DNA. The mode of binding to DNA differs from cisplatin, as
ruthenium complexes form cross-links between DNA strands prob-ably due to
steric hindrance by their octahedral geometry. It has also been shown that
Ru(II) species are much more reactive towards DNA than Ru(III) and Ru(IV)
compounds, the latter two can potentially be seen as less toxic prodrugs.
3. Further medical applications of ruthenium-based complexes
Ruthenium complexes have been under investigation as
immunosuppressants. Cyclosporin A has severe side effects, such as
hypertension, nephrotoxicity and nausea; hence there is a drive to search for
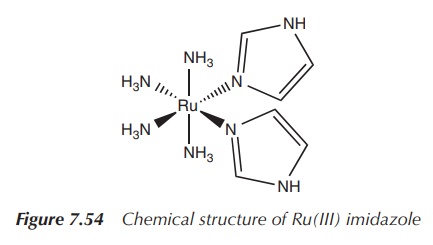
new drugs. Ru(III) imidazole [Ru(NH3)4(Im)2]
is a fairly stable complex that has been shown to inhibit the T-cell
proliferation at nanomolar level (Figure
7.54).
Ruthenium complexes have also shown promising
results when initially tested as antimicrobials and antibi-otics. Especially in
the fight against malaria, new compounds are desperately needed as the
Plasmodium parasite has become resistant to many traditional treatment options,
mainly chloroquine. Research has shown that the Ru(II) chloroquine complex is
significantly more effective, and it is suggested that the uptake of the metal
complex follows a different route. Similar results have been seen when
ruthenium compounds were tested as antibiotics .
Related Topics
