Hypoglycemia
| Home | | Biochemistry |Chapter: Biochemistry : Metabolic Effects of Insulin and Glucagon
Hypoglycemia is characterized by 1) central nervous system (CNS) symptoms, including confusion, aberrant behavior, or coma; 2) a simultaneous blood glucose level equal to or less than 40 mg/dl; and 3) symptoms being resolved within minutes following the administration of glucose.
HYPOGLYCEMIA
Hypoglycemia is
characterized by 1) central nervous system (CNS) symptoms, including confusion,
aberrant behavior, or coma; 2) a simultaneous blood glucose level equal to or
less than 40 mg/dl; and 3) symptoms being resolved within minutes following the
administration of glucose (Figure 23.13). Hypoglycemia is a medical emergency
because the CNS has an absolute requirement for a continuous supply of
bloodborne glucose to serve as fuel for energy metabolism. Transient
hypoglycemia can cause cerebral dysfunction, whereas severe, prolonged
hypoglycemia causes brain death. Therefore, it is not surprising that the body
has multiple overlapping mechanisms to prevent or correct hypoglycemia. The
most important hormone changes in combating hypoglycemia are elevated glucagon
and the catecholamines, combined with the diminished release of insulin.
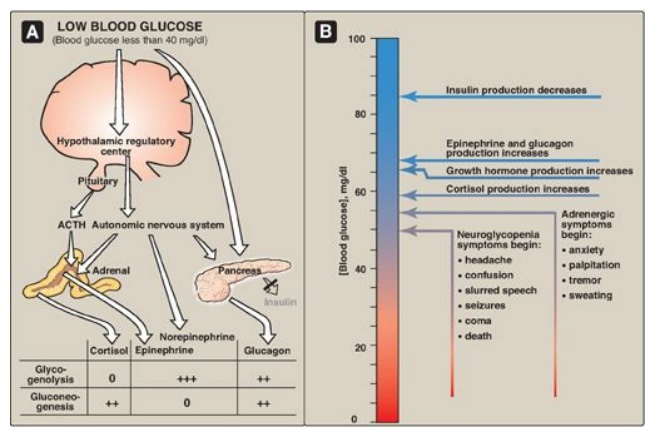
Figure 23.13 A. Actions of
some of the glucoregulatory hormones in response to low blood glucose. B.
Glycemic thresholds for the various responses to hypoglycemia. [Note: Normal
fasted blood glucose is 70-99 mg/100 ml.] + = weak stimulation; ++ = moderate
stimulation; +++ = strong stimulation; 0 = no effect; ACTH =
adrenocorticotropic hormone.
A. Symptoms of hypoglycemia
The symptoms of
hypoglycemia can be divided into two categories. Adrenergic symptoms, such as
anxiety, palpitation, tremor, and sweating, are mediated by catecholamine
release (primarily epinephrine) regulated by the hypothalamus in response to
hypoglycemia. Adrenergic symptoms typically occur when blood glucose levels
fall abruptly. The second category of hypoglycemic symptoms is neuroglycopenic.
Neuroglycopenia (that is, the impaired delivery of glucose to the brain)
results in impairment of brain function, causing headache, confusion, slurred speech,
seizures, coma, and death. Neuroglycopenic symptoms often result from a gradual
decline in blood glucose, often to levels below 40 mg/dl. The slow decline in
glucose deprives the CNS of fuel, but fails to trigger an adequate adrenergic
response.
B. Glucoregulatory systems
Humans have two
overlapping glucose-regulating systems that are activated by hypoglycemia: 1)
the pancreatic α cells, which release glucagon, and 2) receptors in the
hypothalamus, which respond to abnormally low concentrations of blood glucose.
The hypothalamic glucoreceptors can trigger both the secretion of catecholamines
(mediated by the autonomic nervous system) and release of adrenocorticotropic
hormone (ACTH) and growth hormone by the anterior pituitary (see Figure 23.13).
[Note: ACTH increases cortisol synthesis and secretion in the adrenal cortex.]
Glucagon, the catecholamines, cortisol, and growth hormones are sometimes
called the “counterregulatory” hormones because each opposes the action of
insulin on glucose use.
1. Glucagon and epinephrine: Secretion of these hormones is
most important in the acute, short-term regulation of blood glucose levels.
Glucagon stimulates hepatic glycogenolysis and gluconeogenesis. Epinephrine
promotes glycogenolysis and lipolysis, inhibits insulin secretion, and inhibits
the insulin-mediated uptake of glucose by peripheral tissues. Epinephrine
assumes a critical role in hypoglycemia when glucagon secretion is deficient,
for example, in the late stages of type 1 diabetes mellitus. The prevention or
correction of hypoglycemia fails when the secretion of both glucagon and
epinephrine is deficient.
2. Cortisol and growth hormone: These hormones are less important in the short-term maintenance of blood glucose concentrations. They do, however, play a role in the long-term (transcriptional) management of glucose metabolism.
C. Types of hypoglycemia
Hypoglycemia may be
divided into four types: 1) insulin-induced, 2) postprandial (sometimes called
reactive hypoglycemia), 3) fasting hypoglycemia, and 4) alcohol-related.
1. Insulin-induced hypoglycemia: Hypoglycemia occurs frequently in
patients with diabetes who are receiving insulin treatment, particularly those
striving to achieve tight control of blood glucose levels. Mild hypoglycemia in
fully conscious patients is treated by oral administration of carbohydrate.
Unconscious patients are typically given glucagon subcutaneously or
intramuscularly (Figure 23.14).
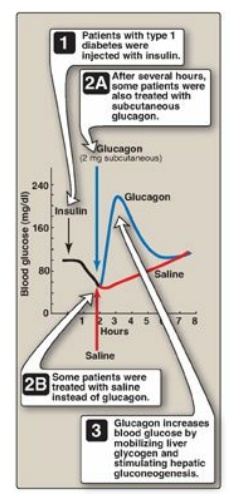
Figure 23.14 Reversal of insulin-induced hypoglycemia by administration of subcutaneous glucagon.
2. Postprandial hypoglycemia: This is the second most common
form of hypoglycemia. It is caused by an exaggerated insulin release following
a meal, prompting transient hypoglycemia with mild adrenergic symptoms. The
plasma glucose level returns to normal even if the patient is not fed. The only
treatment usually required is that the patient eats frequent small meals rather
than the usual three large meals.
3. Fasting hypoglycemia: Low blood glucose during fasting is rare but is more likely to present as a serious medical problem. Fasting hypoglycemia, which tends to produce neuroglycopenic symptoms, may result from a reduction in the rate of glucose production by hepatic glycogenolysis or gluconeogenesis. Thus, low blood glucose levels are often seen in patients with hepatocellular damage or adrenal insufficiency or in fasting individuals who have consumed large quantities of ethanol (see below). Alternately, fasting hypoglycemia may be the result of an increased rate of glucose use by the peripheral tissues due to overproduction of insulin by rare pancreatic tumors. If left untreated, a patient with fasting hypoglycemia may lose consciousness and experience convulsions and coma. [Note: Certain inborn errors of metabolism, for example, defects in fatty acid oxidation, result in fasting hypoglycemia.]
4. Alcohol-related hypoglycemia: Alcohol is metabolized in the
liver by two oxidation reactions (Figure 23.15). Ethanol is first converted to
acetaldehyde by alcohol dehydrogenase. Acetaldehyde is subsequently oxidized to
acetate by aldehyde dehydrogenase (ALDH). [Note: ALDH is inhibited by
disulfiram, a drug that is used in the treatment of chronic alcoholism. The
resulting rise in acetaldehyde results in flushing, tachycardia,
hyperventilation, and nausea.] In each reaction, electrons are transferred to
oxidized nicotinamide adenine dinucleotide (NAD+), resulting in an
increase in the concentration of cytosolic NADH. The abundance of NADH favors
the reduction of pyruvate to lactate and of oxaloacetate (OAA) to malate.
Recall that pyruvate and OAA are intermediates in the synthesis of glucose.
Thus, the ethanol-mediated increase in NADH causes these intermediates of
gluconeogenesis to be diverted into alternate pathways, resulting in the
decreased synthesis of glucose. This can precipitate hypoglycemia, particularly
in individuals who have depleted their stores of liver glycogen. [Note:
Decreased availability of OAA allows acetyl CoA to be diverted to ketone body
synthesis in the liver and can result in alcoholic ketoacidosis.] Hypoglycemia
can produce many of the behaviors associated with alcohol intoxication, such as
agitation, impaired judgment, and combativeness. Therefore, alcohol consumption
in vulnerable individuals (such as those who are fasted or have engaged in
prolonged, strenuous exercise) can produce hypoglycemia that may contribute to
the behavioral effects of alcohol. Becuase alcohol consumption can also
increase the risk for hypoglycemia in patients using insulin, those in an
intensive insulin treatment protocol are counseled about the increased risk of
hypoglycemia that generally occurs many hours after alcohol ingestion. [Note:
Chronic alcohol consumption can also result in alcoholic fatty liver due to
increased hepatic synthesis of TAGs coupled with impaired formation or release
of VLDLs. This occurs as a result of decreased fatty acid oxidation due to a
fall in the NAD+/NADH ratio and increased lipogenesis due to the
increased availability of fatty acids (decreased catabolism) and of
glyceraldehyde 3-phosphate (the dehydrogenase is inhibited by the low NAD+/NADH
ratio). With continued alcohol consumption, alcoholic fatty liver can progress
first to alcoholic hepatitis and then to alcoholic cirrhosis (Figure 23.16).]
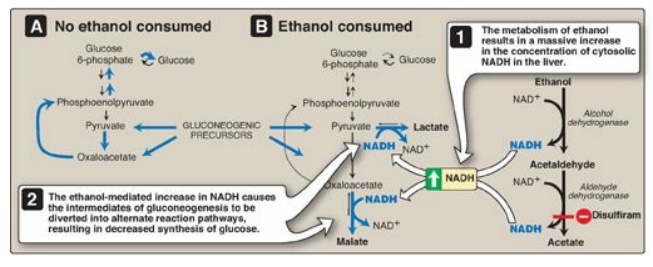
Figure 23.15 A. Normal gluconeogenesis in the absence of ethanol consumption. B. Inhibition of gluconeogenesis resulting from hepatic metabolism of ethanol. NAD(H) = nicotinamide adenine dinucleotide.
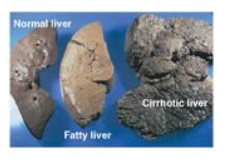
Figure 23.16 Effects of
chronic alcohol consumption on liver morphology.
Related Topics
