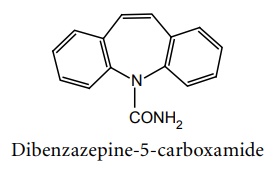
Iminostilbenes
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Anticonvulsants
Anticonvulsants : Iminostilbenes - Synthesis and Drug Profile : Carbamazepine (Tegretol, Zen, Zeptol) - Structure, Properties, uses, Synthesis, Assay, Storage, Dosage forms, Dose | Synthesis and Drug Profile
SYNTHESIS AND DRUG PROFILE
Iminostilbenes
Mode of action: Similar to phenytoin, iminostilbenes limits the repetitive firing of action potential and appears to reduce the rate of recovery of voltage-gated sodium channel from inactivation.
a. Carbamazepine (Tegretol, Zen, Zeptol)
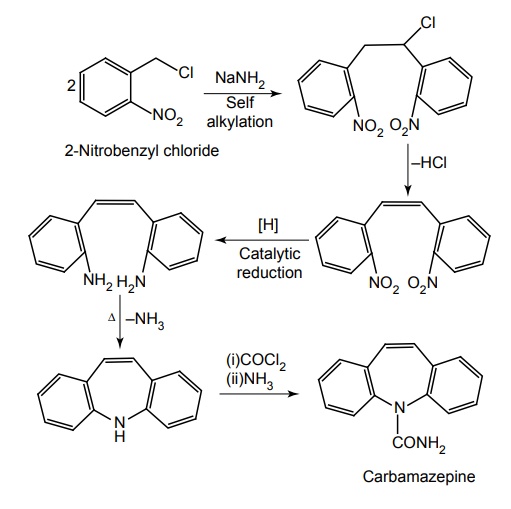
Synthesis
Metabolism: Metabolism proceeds largely through the epoxide form at the cis-stillbene double bond. The epoxide is further converted to 10s and 11s-trans-diol.
Propeties and uses: It is a white or almost white crystalline powder and it shows polymorphism, slightly soluble in water, freely soluble in methylene chloride, but sparingly soluble in acetone and ethanol. Carbamazepine inhibits voltage-dependent sodium channels. Carbamazepine, a urea derivative, is a broad spectrum antiseizure agent, but is toxic, used to treat partial seizures and grandmal seizures. It is also useful in the treatment of pain associated with trigeminal neuralgia.
Assay: It is assayed by adopting liquid chromatography technique. Storage: It should be stored in well-closed airtight containers.
Dosage forms: Carbamazepine tablets B.P.
