Individualization
| Home | | Biopharmaceutics and Pharmacokinetics |Chapter: Biopharmaceutics and Pharmacokinetics : Bioavailability and Bioequivalence
Because of reasonable homogeneity in humans, the dosage regimens are calculated on population basis. However, same dose of a drug may produce large differences in pharmacological response in different individuals.
INDIVIDUALIZATION
Because of reasonable homogeneity in humans, the
dosage regimens are calculated on population basis. However, same dose of a
drug may produce large differences in pharmacological response in different
individuals. This is called as intersubject
variability. In other words, it means
that the dose required to produce a certain response varies from individual to individual. Rational drug
therapy requires individualization of
dosage regimen to fit a particular
patient's needs. This requires knowledge of pharmacokinetics of drugs. The application
of pharmacokinetic principles in the dosage regimen design for the safe and
effective management of illness in individual patient is called as clinical pharmacokinetics.
The two sources of variability in drug responses
are:
1. Pharmacokinetic variability which is due to differences in drug concentration
at the site of action (as reflected from plasma drug concentration) because of interindividual differences in drug absorption,
distribution, metabolism and excretion.
2. Pharmacodynamic variability which is attributed to differences in effect
produced by a given drug concentration.
The major cause for variations is pharmacokinetic
variability. Differences in the plasma
levels of a given drug in the same
subject when given on different occasions is called as intrasubject variability. It is rarely encountered in comparison to
interindividual variations. The
differences in variability differ for different drugs. Some drugs show greater
variability than the others. The major causes of intersubject pharmacokinetic
variability are genetics, disease, age, body-weight and drug-drug interactions.
Less important causes are pharmaceutical formulation, route of administration,
environmental factors and patient noncompliance.
The main objective of individualization is aimed at
optimising the dosage regimen. An inadequate therapeutic response calls for a
higher dosage whereas drug related toxicity calls for a reduction in dosage.
Thus, in order to aid individualization, a drug must be made available in
dosage forms of different dose strengths.
The number of dose strengths in which a drug should be made available depends upon
2 major factors—
1. The therapeutic index of the
drug, and
2. The degree of inter-subject
variability.
Smaller the therapeutic index and greater the
variability, more the number of dose strengths required.
Based on the assumption that all patients require
the same plasma drug concentration range for therapeutic effectiveness, the
steps involved in the individualization of dosage regimen are:
1. Estimation of pharmacokinetic
parameters in individual patient and determining their deviation from the population
values to evaluate the extent of variability. Greater the accountability of
variations, better the chances of attaining the desired therapeutic objective.
2. Attributing the variability to
some measurable characteristic such as hepatic or renal disease, age, weight,
etc.
3. Designing the new dosage
regimen from the collected data.
The design of new dosage regimen involves:
1. Adjustment of dosage, or
2. Adjustment of dosing interval,
or
3. Adjustment of both dosage and
dosing interval.
Dosing of Drugs in Obese Patients
The apparent volume of distribution of a drug is
greatly affected by changes in body weight since the latter is directly related
to the volume of various body fluids. The ideal
body weight (IBW) for men and women can be calculated from following
formulae:
IBW (men) = 50 Kg ± 1 Kg/2.5 cm above or below 150
cm in height (12.17)
IBW (women) = 45 Kg ± 1 Kg/2.5 cm above or below
150 cm in height (12.18)
Any person whose body weight is more than 25% above the IBW is
considered obese. In such patients, the
lean-to-adipose tissue ratio is small because of greater proportion of body fat
which alters the Vd of drugs. The ECF of adipose tissue is small in
comparison to lean tissue in obese patients.
Following generalizations
can be made regarding drug distribution and dose adjustment in obese patients:
1. For drugs such as digoxin that
do not significantly distribute in the excess body space, Vd do not
change and hence dose to be administered should be calculated on IBW basis.
2. For polar drugs such as antibiotics (gentamicin)
which distribute in excess body space of obese patients to an extent less than
that in lean tissues, the dose should be lesser on per Kg total body weight
basis but more than that on IBW basis.
3. In case of drugs such as
caffeine, theophylline, lidocaine and lorazepam which distribute to the same
extent in both lean and adipose tissues, the Vd is larger in obese
patients but same on per Kg total body weight basis; hence, dose should be
administered on total body weight basis.
4. For drugs such a phenytoin,
diazepam and thiopental which are lipid soluble and distribute more in adipose
tissues, the Vd is larger per Kg body weight in obese patients and
hence they require larger doses, more than that on total body weight basis.
Changes in dose based on alteration of Vd
is also attributed to modification of clearance and half-life of the drug.
Dosing of Drugs in Neonates, Infants and Children
The usual dosage regimen calculated on population
basis refers to that for adults. Neonates, infants and children require
different dosages than adults because of differences in body surface area, TBW
and ECF on per Kg body weight basis. The dose for such patients are calculated
on the basis of their body surface area and not on body weight basis because
the body surface area correlates better with dosage requirement, cardiac
output, renal blood flow and glomerular filtration in children. A simple
formula in comparison to DuBois and DuBois for computing surface area (SA) in
square meters is Mosteller’s equation:
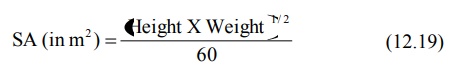
Infants and children require larger mg/Kg doses
than adults because:
1. Their body surface area per Kg
body weight is larger, and hence
2. Larger volume of distribution
(particularly TBW and ECF).
The child’s maintenance dose can be calculated from
adult dose by using the following equation:

where 1.73 is surface area in m2 of an
average 70 Kg adult. Since the surface area of a child is in proportion to the
body weight according to equation 12.21,
SA (in m2) = Body Weight (in Kg)0.7 (12.21)
The following relationship can also be written for
child’s dose:
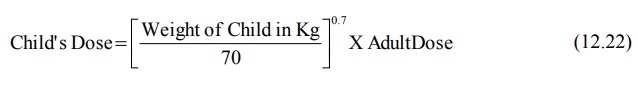
As the TBW in neonates is 30% more than that in
adults,
1. The Vd for most
water-soluble drugs is larger in infants, and
2. The Vd for most
lipid-soluble drugs is smaller.
Accordingly, the dose should be
adjusted.
Dosing of Drugs in Elderly
Drug dose should be reduced in elderly patients
because of a general decline in body function with age. The lean body mass
decreases and body fat increases by almost 100% in elderly persons as compared
to adults. Because of smaller volume of body water, higher peak alcohol levels
are observed in elderly subjects than in young adults. Vd of a
water-soluble drug may decrease and that of a lipid-soluble drug like diazepam
increases with age. Age related changes in hepatic and renal function greatly
alters the clearance of drugs. Because of progressive decrease in renal
function, the dosage regimen of drugs that are predominantly excreted unchanged
in urine should be reduced in elderly patients.
A general equation that allows calculation of
maintenance dose for a patient of any age (except neonates and infants) when
maintenance of same Css,av is desired is:
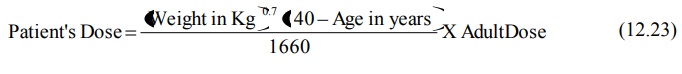
Dosing of Drugs in Hepatic Disease
Disease is a major source of variations in drug
response. Both pharmacokinetics and pharmacodynamics of many drugs are altered
by diseases other than the one which is being treated.
The influence of hepatic disorder on drug
availability and disposition is unpredictable because of the multiple effects
that liver disease produces—effects on drug metabolising enzymes, on drug
binding and on hepatic blood flow. Hence, a correlation between altered drug
pharmacokinetics and hepatic function is often difficult. For example, unlike
excretion, there are numerous pathways by which a drug may be metabolised and
each is affected to a different extent in hepatic disease. Generally speaking,
drug dosage should be reduced in patients with hepatic dysfunction since
clearance is reduced and availability is increased in such a situation.
Dosing of Drugs in Renal Disease
In patients with renal failure, the half-life of a
drug is increased and its clearance drastically decreased if it is
predominantly eliminated by way of excretion. Hence, dosage adjustment should
take into account the renal function of the patient and the fraction of
unchanged drug excreted in urine. One such method was discussed in chapter 6 on Excretion of Drugs.
There are two additional methods for dose
adjustment in renal insufficiency if the Vd change is assumed to be
negligible. These methods are based on maintaining the same average
steady-state concentration during renal dysfunction as that achieved with the
usual multiple dosage regimen and normal renal function. The adjustments are
based on equations 12.6 and 12.7.
1. Dose adjustment based on total body clearance: Rewriting equation 12.6, the parameters
to be adjusted in renal insufficiency are shown below:
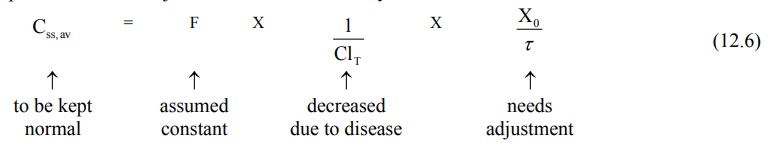
If ClT', Xo' and τ' represent the values for the renal failure patient, then the equation
for dose adjustment is given as:
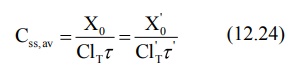
Rearranging in terms of dose and dose interval to
be adjusted, the equation is:
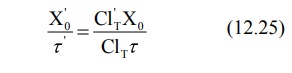
From above equation, the regimen may be adjusted by
reduction in dosage or increase in dosing interval or a combination of both.
2. Dose adjustment based on elimination rate constant or half-life: Rewriting equation 12.7, the parameters to be adjusted in renal
insufficiency are:

If t½', Xo' and τ'
represent the values for the renal failure patient, then:
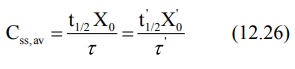
Rearranging the above equation in terms of dose and
dose interval to be adjusted, we get:
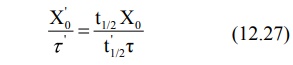
Because of prolongation of half-life of a drug due
to reduction in renal function, the time taken to achieve the desired plateau
takes longer, the more severe the dysfunction. Hence, such patients sometimes
need loading dose.
Related Topics
