Insulin
| Home | | Biochemistry |Chapter: Biochemistry : Metabolic Effects of Insulin and Glucagon
Insulin is a peptide hormone produced by the β cells of the islets of Langerhans, which are clusters of cells that are embedded in the endocrine portion of the pancreas.
INSULIN
Insulin is a peptide
hormone produced by the β cells of the islets of Langerhans, which are clusters
of cells that are embedded in the endocrine portion of the pancreas (Figure
23.2). [Note: “Insulin” is from the Latin for island.] The islets make up only
about 1%– 2% of the total cells of the pancreas. Insulin is the most important
hormone coordinating the use of fuels by tissues. Its metabolic effects are
anabolic, favoring, for example, synthesis of glycogen, triacylglycerols
(TAGs), and protein.
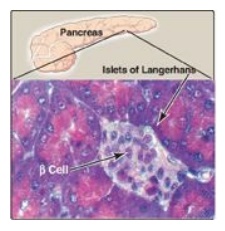
Figure 23.2 Islets of Langerhans.
A. Structure of insulin
Insulin is composed of 51 amino acids arranged in two polypeptide chains, designated A (21 amino acids) and B, which are linked together by two disulfide bridges (Figure 23.3A). The insulin molecule also contains an intramolecular disulfide bridge between amino acid residues of the A chain. [Note: Insulin was the first peptide for which the primary structure was determined, and the first therapeutic molecule made by recombinant DNA technology.]
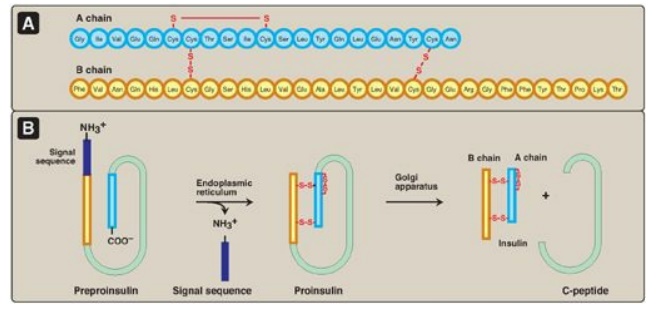
Figure 23.3 A. Structure of insulin. B. Formation of human insulin from preproinsulin.
B. Synthesis of insulin
The processing and transport of intermediates that occur during the synthesis of insulin are shown in Figures 23.3B and 23.4. Biosynthesis involves production of two inactive precursors, preproinsulin and proinsulin, that are sequentially cleaved to form the active hormone plus the connecting or C-peptide (see Figure 23.4). [Note: The C-peptide is essential for proper insulin folding. Also, because its half-life in the plasma is longer than that of insulin, the C-peptide is a good indicator of insulin production and secretion.] Insulin is stored in the cytosol in granules that, given the proper stimulus (see below), are released by exocytosis. Insulin is degraded by insulin-degrading enzyme, which is present in the liver and, to a lesser extent, in the kidneys. Insulin has a plasma half-life of approximately 6 minutes. This short duration of action permits rapid changes in circulating levels of the hormone.
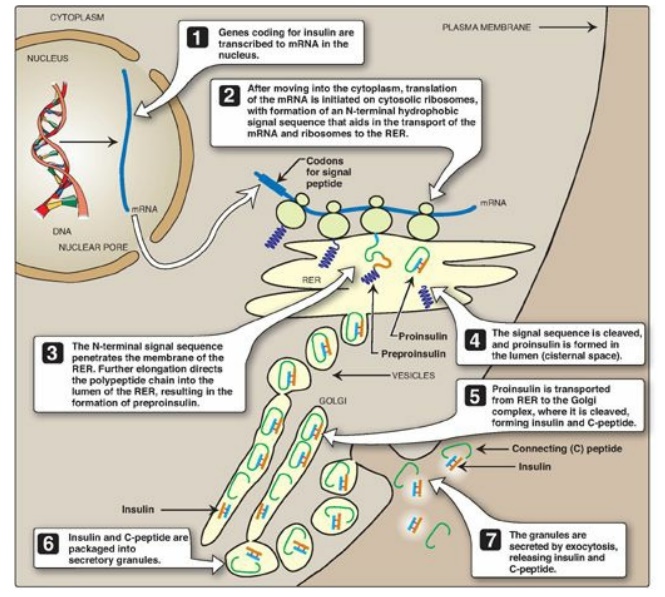
Figure 23.4 ntracellular movements of insulin and its precursors. mRNA = messenger RNA; RER = rough endoplasmic reticulum.
C. Regulation of insulin secretion
Secretion of insulin is
regulated by bloodborne fuels and hormones.
1. Stimulation of insulin secretion: Insulin secretion by the pancreatic β cells is closely coordinated with the release of glucagon by pancreatic α cells (Figure 23.5). The relative amounts of insulin and glucagon released by the pancreas are regulated so that the rate of hepatic glucose production is kept equal to the use of glucose by peripheral tissues. In view of its coordinating role, it is not surprising that the β cell responds to a variety of stimuli. In particular, insulin secretion is increased by glucose, amino acids, and gastrointestinal peptide hormones.
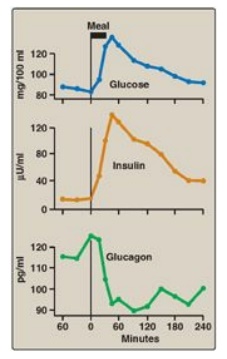
Figure 23.5 Changes in blood levels of glucose, insulin, and glucagon after ingestion of a carbohydrate-rich meal.
a. Glucose: Ingestion of a carbohydrate-rich meal leads to a rise in blood glucose, the primary stimulus for insulin secretion (see Figure 23.5). The β cells are the most important glucose-sensing cells in the body. Like the liver, β cells contain GLUT-2 transporters and express glucokinase (hexokinase IV;). At blood glucose levels above 45 mg/dl, glucokinase phosphorylates glucose in amounts proportional to the glucose concentration. Proportionality results from the lack of direct inhibition of glucokinase by glucose 6-phosphate, its product. Additionally, the sigmoidal relationship between the velocity of the reaction and substrate concentration maximizes the enzyme’s responsiveness to changes in blood glucose level. Metabolism of glucose 6-phosphate generates adenosine triphosphate (ATP), leading to insulin secretion (see blue box below).
b. Amino acids: Ingestion of protein causes a transient rise in
plasma amino acid levels (for example, arginine) that enhances the
glucose-stimulated secretion of insulin. [Note: Fatty acids have a similar
effect.]
c. Gastrointestinal peptide hormones: The intestinal peptides
glucagon-like protein-1 (GLP-1) and gastric-inhibitory polypeptide ([GIP]; also
called glucose-dependent insulinotropic peptide) increase the sensitivity of β
cells to glucose. They are released from the small intestine after the
ingestion of food, causing an anticipatory rise in insulin levels and, thus,
are referred to as “incretins.” Their action may account for the fact that the
same amount of glucose given orally induces a much greater secretion of insulin
than if given intravenously (IV).
Glucose-dependent release of insulin into blood is mediated through a rise in calcium (Ca2+) concentration in the β cell. Glucose taken into β cells is phosphorylated and metabolized, with subsequent production of ATP. ATP-sensitive potassium (K+) channels close, causing depolarization of the plasma membrane, opening of voltage-gated Ca2+ channels, and influx of Ca2+ into the cell. Ca2+ causes vesicles containing insulin to be exocytosed from the β cell. Sulfonylureas, oral agents used to treat type 2 diabetes, increase insulin secretion by closing ATP-sensitive K+ channels.
2. Inhibition of insulin secretion: The synthesis and release of
insulin are decreased when there is a scarcity of dietary fuels and also during
periods of physiologic stress (for example, infection, hypoxia, and vigorous
exercise). These effects are mediated primarily by the catecholamines
norepinephrine and epinephrine, which are made from tyrosine in the sympathetic
nervous system and the adrenal medulla and secreted. Secretion is largely
controlled by the nervous system. The catecholamines (primarily epinephrine)
have a direct effect on energy metabolism, causing a rapid mobilization of
energy-yielding fuels, including glucose from the liver (produced by
glycogenolysis or gluconeogenesis;) and fatty acids from adipose tissue
(produced by lipolysis;). In addition, these biogenic amines can override the
normal glucose-stimulated release of insulin. Thus, in emergency situations,
the sympathetic nervous system largely replaces the plasma glucose
concentration as the controlling influence over β-cell secretion. The
regulation of insulin secretion is summarized in Figure 23.6.
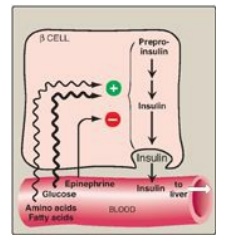
Figure 23.6 Regulation of insulin release from pancreatic β cells. [Note: Gastrointestinal peptide hormones also stimulate insulin release.]
D. Metabolic effects of insulin
Insulin promotes the
storage of nutrients as glycogen, TAG, and protein and inhibits their
mobilization.
1. Effects on carbohydrate metabolism: The effects of insulin on glucose
metabolism promote its storage and are most prominent in three tissues: liver,
muscle, and adipose. In the liver and muscle, insulin increases glycogen
synthesis. In the muscle and adipose, insulin increases glucose uptake by
increasing the number of glucose transporters (GLUT-4;) in the cell membrane.
The IV administration of insulin, thus, causes an immediate decrease in blood glucose
level. In the liver, insulin decreases the production of glucose through the
inhibition of glycogenolysis and gluconeogenesis. [Note: The effects of insulin
are due not just to changes in enzyme activity, but also in enzyme amount
insofar as insulin affects gene transcription.]
2. Effects on lipid metabolism: Adipose tissue responds rapidly to
a rise in insulin, which causes a significant reduction in the release of fatty
acids by inhibiting the activity of hormone-sensitive lipase, which degrades
lipids in adipose tissue. Insulin acts by promoting the dephosphorylation and,
hence, inactivation of the enzyme. Insulin also increases the transport and
metabolism of glucose into adipocytes, providing the glycerol 3-phosphate
substrate for TAG synthesis. Expression of the gene for lipoprotein lipase is
increased by insulin in adipose tissue, thereby providing fatty acids for
esterification to the glycerol. [Note: Insulin also promotes the conversion of
glucose to TAG in the liver. The TAGs are secreted in very-low-density
lipoproteins (VLDLs).]
3. Effects on protein synthesis: In most tissues, insulin
stimulates the entry of amino acids into cells and protein synthesis. [Note:
Insulin stimulates protein synthesis through activation of factors required for
translation initiation.]
E. Mechanism of insulin action
Insulin binds to
specific, high-affinity receptors in the cell membrane of most tissues,
including liver, muscle, and adipose. This is the first step in a cascade of
reactions ultimately leading to a diverse array of biologic actions (Figure
23.7).
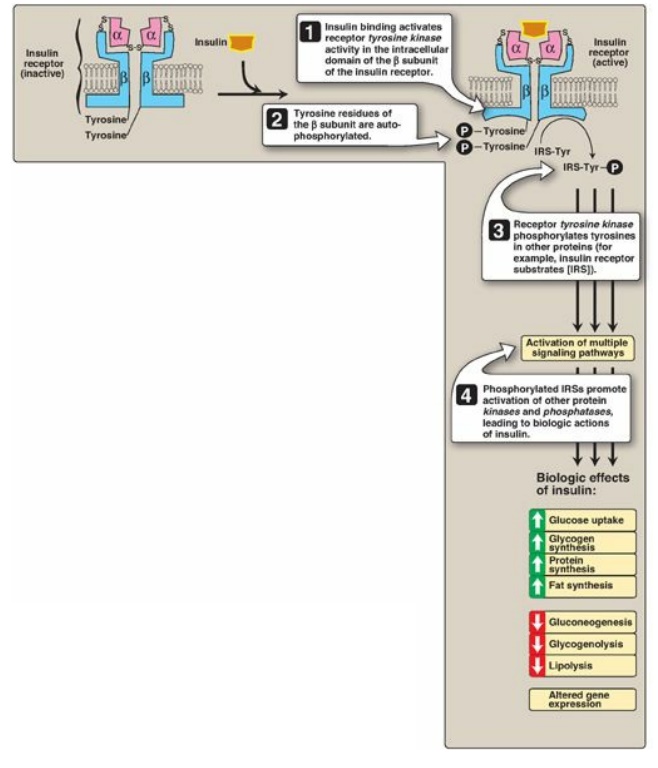
Figure 23.7 Insulin receptor. P = phosphate; Tyr = tyrosine.
1. Insulin receptor: The insulin receptor is
synthesized as a single polypeptide that is glycosylated and cleaved into α and
β subunits, which are then assembled into a tetramer linked by disulfide bonds
(see Figure 23.7). The extracellular α subunit contains the insulin-binding
site. A hydrophobic domain in each β subunit spans the plasma membrane. The
cytosolic domain of the β subunit is a tyrosine kinase, which is activated by
insulin. As a result, the insulin receptor is classified as a tyrosine-kinase
receptor.
2. Signal transduction: The binding of insulin to the α subunits of the insulin receptor induces conformational changes that are transmitted to the β subunits. This promotes a rapid autophosphorylation of specific tyrosine residues on each β subunit ( s e e Figure 23.7). Autophosphorylation initiates a cascade of cell-signaling responses, including phosphorylation of a family of proteins called insulin receptor substrates (IRSs). At least four IRSs have been identified that show similar structures but different tissue distributions. Phosphorylated IRS proteins interact with other signaling molecules through specific domains, activating a number of pathways that affect gene expression, cell metabolism, and growth. The actions of insulin are terminated by dephosphorylation of the receptor.
3. Membrane effects of insulin: Glucose transport in some tissues,
such as muscle and adipose, increases in the presence of insulin (Figure 23.8).
Insulin promotes movement of insulin-sensitive glucose transporters (GLUT-4s)
from a pool located in intracellular vesicles to the cell membrane. [Note:
Movement is the result of a signaling cascade in which an IRS binds to and
activates a kinase (phosphoinositide 3-kinase), leading to phosphorylation of
the membrane phospholipid phosphatidylinositol 4,5-bisphosphate to the
3,4,5-trisphosphate form that binds to and activates phosphoinositide kinase 1.
This kinase, in turn, activates Akt (protein kinase B), resulting in GLUT-4
movement.] In contrast, other tissues have insulin-insensitive systems for
glucose transport (Figure 23.9). For example, hepatocytes; erythrocytes; and
cells of the nervous system, intestinal mucosa, renal tubules, and cornea do
not require insulin for glucose uptake.
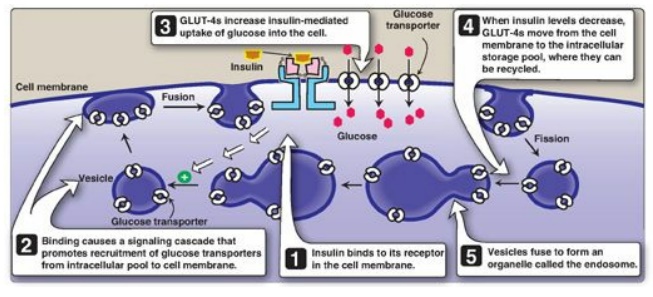
Figure 23.8 Insulin-mediated recruitment of glucose transporters (GLUT-4s) from intracellular stores in skeletal and cardiac muscle and adipose tissue.
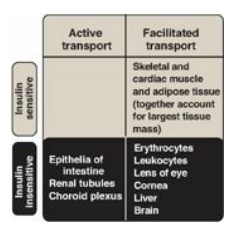
Figure 23.9 Characteristics of glucose transport in various tissues.
4. Receptor regulation: Binding of insulin is followed by
internalization of the hormone–receptor complex. Once inside the cell, insulin
is degraded in the lysosomes. The receptors may be degraded, but most are
recycled to the cell surface. [Note: Elevated levels of insulin promote the degradation
of receptors, therby decreasing the number of surface receptors. This is one
type of “downregulation.”]
5. Time course of insulin actions: The binding of insulin provokes a wide range of actions. The most immediate response is an increase in glucose transport into adipocytes and skeletal and cardiac muscle cells that occurs within seconds of insulin binding to its membrane receptor. Insulin-induced changes in enzymic activity in many cell types occur over minutes to hours and reflect changes in the phosphorylation states of existing proteins. Insulin-induced increase in the amount of many enzymes, such as glucokinase, liver pyruvate kinase, acetyl coenzyme A (CoA) carboxylase (ACC), and fatty acid synthase, requires hours to days. These changes reflect an increase in gene expression through increased transcription (mediated by sterol regulatory element–binding protein-1;) and translation.
Related Topics
