Large Trials of Cox-2 Inhibitors in Disease Prevention
| Home | | Pharmacovigilance |Chapter: Pharmacovigilance: NSAIDs - COX-2 Inhibitors – Risks and Benefits
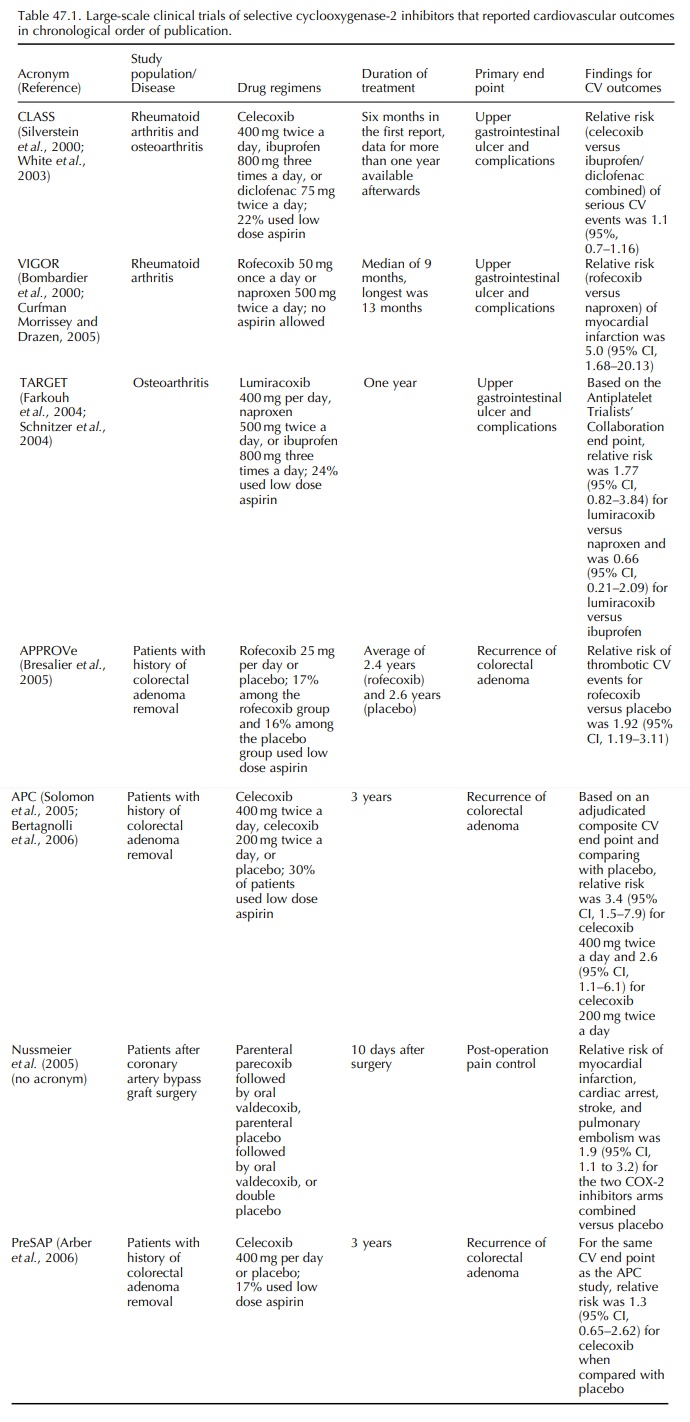
The study that prompted the market withdrawal of rofecoxib was the Adenomatous Polyp Preven-tion on Vioxx (APPROVe) Trial.
LARGE TRIALS OF COX-2 INHIBITORS
IN DISEASE PREVENTION (TABLE 47.1)
COX-2 INHIBITORS AND PREVENTION OF COLORECTAL ADENOMA
Rofecoxib in APPROVe
The
study that prompted the market withdrawal of rofecoxib was the Adenomatous
Polyp Preven-tion on Vioxx (APPROVe) Trial (Bresalier et al., 2005). The trial was funded by the manufacturer of
rofecoxib and the primary objective of the trial was to evaluate the efficacy
of long-term rofecoxibuse in the prevention of adenomatous polyps recur-rence
among patients with a history of colorectal adenomas. Patients who had history
of removal of histologically confirmed colorectal adenoma were randomly
assigned to receive rofecoxib 25 mg per day or placebo for three years.
Exclusion criteria included patients who had prior history of coronary heart
disease and need for long-term NSAID therapy. Patient enrollment started before
the adverse cardio-vascular outcomes from VIGOR became available and patient
enrollment completed in November 2001. The original protocol specified that
patients on low dose aspirin (less than 100 mg per day) would be excluded, but
after the VIGOR results became available, enrolled patients were allowed to
take aspirin less than 100 mg per day. A committee blinded to treat-ment
assignment evaluated all cardiovascular events and the composite cardiovascular
end point was fatal and non-fatal myocardial infarction, unstable angina,
sudden death from cardiac causes, fatal and non-fatal ischemic stroke,
transient ischemic attack, peripheral arterial thrombosis, peripheral venous
thrombosis, and pulmonary embolism. At an interim analysis that was conducted
in September 2004 when 72 patients (46 among the rofecoxib group and 26 among the
placebo group) had confirmed thrombotic events, the data and safety monitoring
board found an increased risk of cardiovascular events among the rofecoxib arm,
with a relative risk of 1.92 (95% CI, 1.19– 3.11). The same conclusion could be
reached if the APTC definition for cardiovascular end point was used. Although
the Kaplan–Meier curves of adverse cardiovascular outcomes for the two
treatment arms did not diverge after 18 months, there was insuffi-cient
statistical power to evaluate the risk difference during the first 18 months of
treatment and no defini-tive conclusion could be made about when the risk might
increase after initiation of rofecoxib therapy (Lagakos, 2006). APPROVe was
terminated on 30 September 2004 and rofecoxib was withdrawn from the worldwide
market on the same day.
Celecoxib in APC and PreSAP
Results
of the APPROVe trial prompted the National Cancer Institute to carry out a
cardiovascular safety analysis to evaluate the cardiovascular effects of
celecoxib in the Adenoma Prevention with Cele-coxib (APC) study (Solomon et al., 2005). Similar to APPROVe, APC
was a chemoprevention trial that evaluated the efficacy of a COX-2 inhibitor in
the prevention of recurrence of colorectal polyp. It was co-sponsored by the
National Cancer Institute and the manufacturer of celecoxib. Subjects were
randomly assigned to receive celecoxib 400 mg two times per day, celecoxib 200
mg two times per day, or placebo. Prior history of cardiovascular disease was
not an exclusion criterion. Subject enrollment was completed in March 2002 and
the treatment phase of the trial was terminated on 16 December 2004 because of
cardiovascular safety concerns. The safety commit-tee evaluated a composite
cardiovascular end point of myocardial infarction, stroke, congestive heart
fail-ure, and death due to cardiovascular disease during the three-year
follow-up period. An increased cardio-vascular risk was found among patients
who received celecoxib 800 mg per day when compared with the placebo arm
(relative risk 3.4; 95% CI, 1.5–7.9). For the comparison between the groups who
received celecoxib 400 mg per day and placebo, the relative risk was 2.6 (95%
CI, 1.1–6.1) (Bertagnolli et al.,
2006).
The
manufacturer of celecoxib funded another chemoprevention trial of colorectal
polyps called Prevention of Colorectal Sporadic Adenomatous Polyps (PreSAP)
trial that compared the efficacy of celecoxib 400 mg daily and placebo in the
preven-tion of colorectal adenoma recurrence. Preliminary findings were
reported at the FDA advisory commit-tee meeting in February 2005 (Levin, 2005)
and the final report showed that for the same compos-ite end point used in APC,
relative risk for use of celecoxib 400 mg per day as compared with placebo was
1.3 (95% CI, 0.65–2.62) (Arber et al.,
2006).
Due
to the cardiovascular safety signals discov-ered from preliminary analysis of
APC and PreSAP, the National Cancer Institute commissioned a cardio-vascular
safety committee to combine cardiovascular safety data from APC and PreSAP and
use a single set of criteria to blindly adjudicate cardiovascular end points
(Solomon et al., 2006). The overall
relative risk was 1.9 (95% CI, 1.1–3.1) for all celecoxib doses when compared
with placebo in these two colorectal adenoma prevention trials.
CELECOXIB AND NAPROXEN IN AN ALZHEIMER’S DISEASE PREVENTION TRIAL
The
US National Institute of Aging sponsored the Alzheimer’s Disease
Anti-inflammatory Prevention Trial (ADAPT) that began patient recruitment in
2001. Subjects of age 70 or older, but who did not have symptoms of dementia,
were randomly assigned to receive long-term use of celecoxib 200 mg twice a
day, naproxen 220 mg twice a day, or placebo. At an interim cardiovascular
safety analysis conducted in December 2004, naproxen use was found to be
associated with increased risk of adverse cardiovas-cular or cerebrovascular
events when compared with the placebo group. No increased risk in the celecoxib
group in comparison with placebo was found. The National Institute of Health
announced that the trial was suspended, but no data were reported in
peer-reviewed journals (NIH News Release, 2004).
Related Topics
