Mass Transfer in Turbulent and Laminar Flow
| Home | | Pharmaceutical Technology |Chapter: Pharmaceutical Engineering: Mass Transfer
When a fluid flows over a surface, the surface retards the adjacent fluid region, forming a boundary layer.
MASS TRANSFER IN TURBULENT AND
LAMINAR FLOW
As
already explained, movement of molecules across the streamlines of a fluid in
laminar flow can only occur by molecular diffusion. If the concentration of a
component, A, varies in a direction normal to the streamlines, the molar rate
of diffusion will be given by equation (4.1).
When
a fluid flows over a surface, the surface retards the adjacent fluid region,
forming a boundary layer. If flow throughout the fluid is laminar, the equation
for molecular diffusion may be used to evaluate the mass transferred across the
boundary layer. In most important cases, however, flow in the bulk of the fluid
is turbulent. The boundary layer is then considered to consist of three
distinct flow regimes. In the region of the boundary layer most distant from
the surface, flow is turbulent and mass transfer is the result of the
interchange of large portions of the fluid. Mass interchange is rapid, and
concentration gra-dients are low. As the surface is approached, a transition
from turbulent to laminar flow occurs in the transition or buffer region. In
this region, mass transfer by eddy diffusion and molecular diffusion are of
comparable magnitude. In a fluid layer at the surface, a fraction of a millimeter
thick, laminar flow conditions persist. This laminar sublayer, in which
transfer occurs by molecular diffusion only, offers the main resistance to mass
transfer, as shown in Figure 4.2.
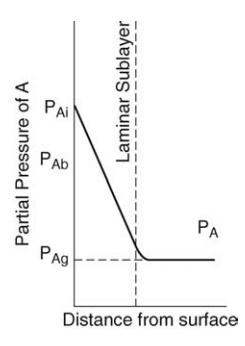
FIGURE 4.2 Mass transfer at a boundary.
As
flow becomes more turbulent, the thickness of the laminar sublayer and its
resistance to mass transfer decrease.
One
approach to the evaluation of the rate of mass transfer under these conditions
lies in the postulation of a film, the thickness of which offers the same
resistance to mass transfer as the combined laminar, buffer, and turbulent
regions. The analogy with heat transfer by conduction and convection is exact,
and quantitative relations between heat and mass transfer can be developed for
some situations. This, however, is not attempted in this text. The postulate of
an effective film is explained by reference to Figure 4.2.
A
gas flows over a surface, and equimolecular counterdiffusion of com-ponents A
and B occurs, A away from the surface and B toward the surface. The variation
in partial pressure of A with distance from the surface is shown in the figure.
At the surface, the value is PAi. A linear fall to PAb
occurs over the laminar sublayer. Beyond this, the partial pressure falls less
steeply to the value PA at the edge of the boundary layer. PAg,
a value slightly higher than this, is the average partial pressure of A in the
entire system. In general, the gas content of the laminar layer is so small
that PA and PAg are virtually equal. If molecular
diffusion were solely responsible for diffusion, the partial pressure, PAg,
would be reached at some fictitious distance, x0, from the surface,
over which the concentration gradient (PAi – PAg)/x’ exists. The molar
rate of mass transfer would then be
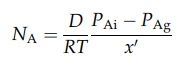
x' is not known, however, and this equation may be written as
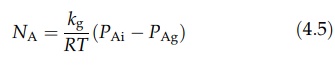
where
kg, is a mass transfer coefficient, the unit of which is m/sec.
Since CA = PA/RT, we can also write
NA
= kg(CAi - CAg)
where
CAi and CAg are the gas concentrations at either side of
the film. Similar equations describe the diffusion of B in the opposite
direction.
Diffusion
across a liquid film is described by the equation
NA
= k1(CAi CAl) (4:6)
where
CAi is the concentration of component A at the interface and CAl
is its concentration in the bulk of the phase.
In
all cases, the mass transfer coefficient will depend on the diffusivity of the
transferred material and the thickness of the effective film. The latter is
largely determined by the Reynolds number of the moving fluid, that is, its
average velocity, its density, its viscosity, and some linear dimension of the
system. Dimensional analysis will give the following relation:
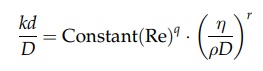
where
Re is the Reynolds number, k is the mass transfer coefficient, D is the
diffusivity, and d is a dimension
characterizing the geometry of the system.
This
relation is analogous to the expression for heat transfer by forced convection
given in chapter 3. The dimensionless group kd/D
corresponds to the Nusselt group in heat transfer. The parameter η/ρD is known as the Schmidt number and
is the mass transfer counterpart of the Prandtl number. For example, the
evaporation of a thin liquid film at the wall of a pipe into a turbulent gas is
described by the equation
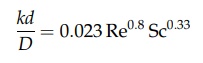
where
Sc is the Schmidt number. Although the equation expresses experimental data,
comparison with equation (13) from the heat transfer section again
dem-onstrates the fundamental relation of heat and mass transfer.
Similar
relations have been developed empirically for other situations. The flow of
gases normal to and parallel to liquid surfaces can be applied to drying
processes, and the agitation of solids in liquids can provide information for
crystallization or dissolution. The final correlation allows the estimation of
the mass transfer coefficient with reasonable accuracy.
Related Topics
