Matching Nucleophiles with Electrophiles
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Carbon-Carbon Bond Formation Between Carbon Nucleophiles and Carbon Electrophiles
Having defined nucleophilic and electrophilic carbon species and having learned to produce a variety of them, the next step is to match the reactivities of the nucleophiles and electrophiles so that carbon–carbon bond formation can occur in a controllable and selective fashion.
MATCHING NUCLEOPHILES WITH
ELECTROPHILES
Having
defined nucleophilic and electrophilic carbon species and having learned to
produce a variety of them, the next step is to match the reactivities of the
nucleophiles and electrophiles so that carbon–carbon bond formation can occur
in a controllable and selective fashion. Qualitatively the order of
reactivities for nucleophiles and electrophiles used in carbon–carbon
bond-forming reactions are shown below. In general, many of the most useful
carbon–carbon bond-forming reactions take place with nucleophiles and
electrophiles in the middle ranges of reactivity. Highly reactive electrophiles
and nucleophiles are often difficult to control while nucleophiles and
electrophiles of low reactivity often fail to react effectively. Nevertheless
it is reactivity matching that is most important in producing useful reactions.
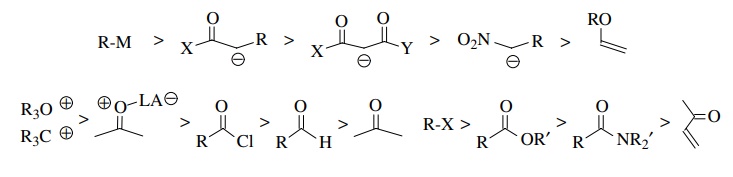
When
stabilized (and consequently less reactive) anions are employed as the
nucleophile, more reactive electrophiles are needed for successful
carbon–carbon bond formation. Nitronate anions, which are highly resonance
stabilized, fail to react with simple alkyl halide electrophiles. On the other
hand, β-dicarbonyl compounds react
effectively with primary and some secondary alkyl bromides and iodides to give
monoalkylated products.
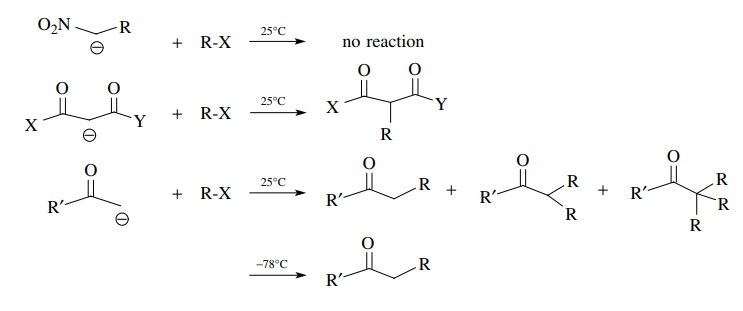
Under
the same conditions simple enolates react vigorously with alkyl halides (which
must be primary) to give mono- and polyalkylated products. The reactivity of
the simple enolate is greater and cannot be controlled at room temperature.
However, if the alkylation is carried out at low temperature, the reaction can
be controlled and smooth monoalkylation of simple enolates can be achieved. The
same is true for the alkylation of acetylide anions, which must be carried out
at low temperature for successful alkylation.
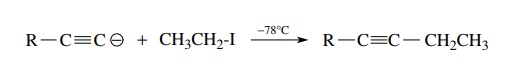
Related Topics
