Mechanisms of Biofilm Tolerance
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Microbial Biofilms: Consequences For Health
It is now believed that the tolerance to antimicrobials displayed by biofilms is a multifactorial process involving, to some degree or another, a number of different mechanisms contributing to the survival of the population, if not the individual cell.
MECHANISMS OF BIOFILM TOLERANCE
It is now believed that
the tolerance to antimicrobials displayed by biofilms is a multifactorial
process involving, to some degree or another, a number of different mechanisms
contributing to the survival of the population, if not the individual cell. A
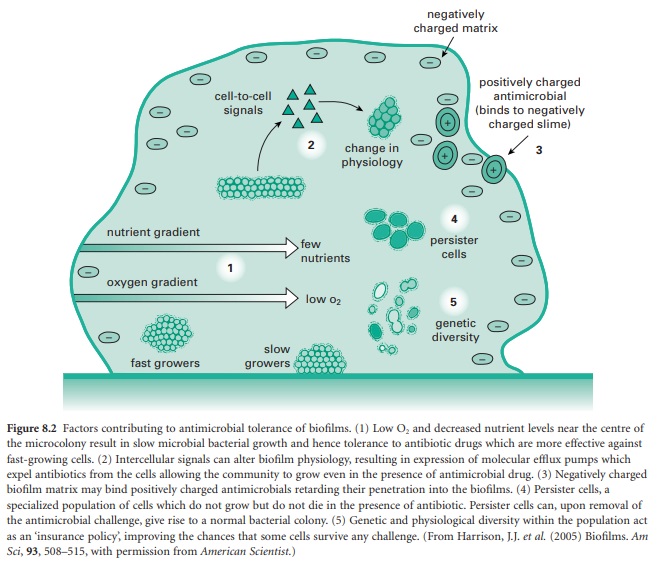
model for this multifactorial tolerance is shown in Figure 8.2. Contributing to
the tolerance of biofilms are factors ranging from the structural components of
the biofilm, the physiological potential of cells spread throughout the biofilm
and the expression of the genetic phenotypes of disparate populations of cells,
all derived from the original clonal population(s) that made up the biofilm.
1) Biofilm structure
The hypothesis that the
extracellular matrix acts as the gatekeeper for the penetration of
antimicrobials into the biofilm, as shown in point 3 in Figure 8.2, has
engendered many studies and a great deal of controversy. When biofilms were
first visualized using both transmission and scanning electron microscopy, the
dehydrated matrix seen in these original micrographs led to the belief that
biofilms were very flat and dense structures where the compact and highly
charged matrix around the biofilm would prevent penetration of antibiotics into
the biofilm; hence this diffusion barrier would render them resistant to
antimicrobial treatment. Stabilization of the matrix and cross sections through
the biofilm revealed a very different picture of the biofilm, where cells were
seen to exist within a very hydrated matrix containing channels to allow for
nutrient transfer into the biofilm and the diffusion of waste out. The matrix
is now believed to be composed of bacterially derived carbohydrate, the composition
of which is dependent upon the bacterial species, nutrient availability and the
growth conditions of the biofilm. Recently it has been established that DNA is
an important component of the matrix, which may be specifically transported
into the matrix. The role of DNA in the matrix is only now being deciphered. It
has been shown to play a role in the conformation of the carbohydrate and
hypothesized to serve as a gene pool for the diversity seen within the biofilm.
The highly anionic charge of this matrix could be hypothesized to still play an
important role in preventing charged antibiotics from effectively entering the
biofilm and thereby still act as a primary inhibitor of antibiotic killing, as
was originally proposed. Several studies of antibiotic penetration into biofilms
demonstrated that the charge of the antibiotic could affect its penetration.
For example, fluoroquinolones (ciprofloxacin, ofloxacin) that are not highly
charged easily penetrate the matrix, while the penetration of charged aminoglycosides
(tobramycin, gentamicin) is delayed. These studies have not, however, resolved
the issue of the importance of the matrix in the resistance of biofilms. The
rapid entry of fluoroquinolones, for example, may be only into water channels
of the biofilm and not into areas where cells of the biofilm are found, while
the delay in entry of aminoglycosides may affect the rate of entry but may not
affect final concentration significantly enough to alter susceptibility of the
biofilm. Further, penetration of antimicrobials alone may not be as key an
issue as the physiological state of the cells, which is also affected by the
structure and organization of the biofilm. The diffusion into the biofilm of
multiple factors, not limited to just the antibiotics themselves, may impact
the biofilm’s physiological status, thereby affecting the efficacy of
antibiotics against the biofilm.
2) Biofilm physiology
The physiological state
of the biofilm is also affected by its organizational structure, as diffusion
of oxygen, nutrients and waste will ultimately affect all properties associated
with growth and sustainability of the biofilm (shown in part 1 of Figure 8.2).
Sophisticated experiments based on microelectrode probing of the biofilm and
confirmed by dye distribution confocal microscopy assays have established the presence
of oxygen and pH gradients within the biofilm. Gradients of nutrients and end
products are also implicated in defining the different growth properties
throughout the biofilm, which, as described above, has been linked to
antibiotic susceptibility. The hypothesis predicts that antibiotics dependent
on cell growth for activity will be less effective against biofilms because of
the variability of growth within the biofilm. However biofilms, prove to be as
recalcitrant to antibiotics that are not dependent upon cell growth as to those
that do. Further, live dead staining of biofilms does not always correlate with
cell death occurring most rapidly at the outer edges of the biofilm, where
nutrient and oxygen levels are at their highest and hence where growth should
be most rapid. In mixed species biofilms, where each component of the
population may exist within its own niche for optimal growth, this model
becomes even more complex to understand. The complexity of these interactions
will make dissecting the process of antimicrobial tolerance more challenging.
One can, however, argue that targeting one member of the mixed population
within the biofilm may alter the susceptibility patterns of other species that
are dependent upon the symbiotic interactions within the biofilm.
Another focus of study
in biofilm resistance related to spatial orientation and physiology has been to
look at how biofilms deal with oxidative stress. The mechanism of killing of
many antimicrobials, including antibiotics, biocides and metals, is often
associated with redox reactions involving various cellular components. These
reactions may oxidize sensitive cellular thiol (RSH) groups or result in the
production of reactive oxygen species (ROS), such as superoxides, hydroxy radicals,
or hydrogen peroxide. The regulation of antioxidant pathways within the cell,
such as glutathione (GSH) and thioredoxin pathways that manage thiol-disulfide
homeostasis, and the oxyRS, soxRS and marR regulons that render cells resistant to ROS, play an important role in the susceptibility of biofilms to
antimicrobials. The difference in oxygen tension in the biofilm and of the
cells’ response to oxygen stress may prove vital in survival of biofilms to
naturally occurring antibiotics or those used in patient treatment. The role of
the redox potential of E. coli to
metals has demonstrated a very complex picture of these interactions and has
shown a difference in mechanisms between planktonic and biofilm populations.
3) Cellular signalling and biofilm resistance
Our perspective of the
microbial lifestyle has changed from one where bacteria exist mainly as
solitary independent planktonic populations to one where bacteria form adherent
communal populations of bacteria organized into microcolonies called biofilms. This
shift in lifestyle suggests the presence of specific signalling between cells
to allow them to organize these complex structures. Many different genes have
been identified that can alter biofilm formation or antimicrobial
susceptibility, but two global signalling pathways have come to the forefront
as biofilm regulators in many different species of bacteria (see point 2 in
Figure 8.2). Although models of biofilm formation have been proposed that do
not require cell signal molecules, the importance of the following molecules in
biofilm formation and antimicrobial resistance is well established and has even
led to attempts to develop signal antagonists for treatment of biofilm disease
or to create greater efficacy of existing antimicrobials by returning biofilms
to a planktonic like level of susceptibility.
a) Quorum sensing
Quorum sensing (QS) has
been recognized as a key regulatory process associated with biofilm formation
and antibiotic susceptibility. Well studied in Vibrio fischeri, QS involves an enzyme Luxl that produces a small
signalling molecule, or autoinducer,
that diffuses out of the cell. Upon reaching a threshold concentration the
autoinducer will diffuse back into the cell, where cellular transcription is altered
when the autoinducer binds the transcription regulator LuxR and initiates QSspecific
gene expression. In Gram-negative organisms the autoinducer is typically an
acyl homoserine lactone (AHL), but in some organisms multiple QS systems exist.
For example, in Ps. aeruginosa
signalling involves interactions of two distinct AHL compounds, produced by the
Luxl homologues Lasl and Rhll respectively, that interact with their cognate
receptors LasR and RhlR. Yet a third signal system, PQS, is also active in Ps. aeruginosa. In Gram-positive
bacteria QS is typically carried out by autoinducing peptides. As QS is an
integral step in biofilm formation and antibiotic tolerance, it has become a
target for new therapeutics. Inhibitors of the QS signal pathway, assayed for
their ability to either block biofilm formation or the expression of QS-dependent
genes, may provide new approaches to treatment of biofilm disease.
b) Cyclic diguanylate
The universal use of
nucleotides as signalling molecules is well recognized in biology. The
importance of cyclic diguanylate (cdiGMP) as a switch to move bacteria from a
motile planktonic lifestyle to that of an adherent biofilm is now just being
systematically explored. As with other nucleotide regulation systems, two
components are involved in the regulatory pathway. The first is responsible for
the synthesis of the signal, in this case a diguanylate cyclase (DGC) defined
by proteins expressing GGDEF domains. The second component, a phosphodiesterase
(PDE), degrades the active signal and is associated with two distinct domains,
EAL and HDGYP. The high level of redundancy of these domains makes understanding
the mechanisms by which cdiGMP regulates biofilm formation and virulence a
complex issue, where patterns of temporal and spatial separation remain to be resolved.
4) Plasticity of biofilms
The final mechanism by
which biofilms become less susceptible to antimicrobials is sketched in parts 4
and 5 of Figure 8.2. Biofilms are able to give rise to unique subpopulations;
in some cases these may be part of the normal diverse metabolic activity found
within the nutrient and oxygen gradients of the biofilm, an example being
persister cell populations, or they may be subpopulations that are derived in
response to stress but which are not classically resistant populations
according to definitions discussed previously. Being part of a population, as
opposed to being a single cell, allows for the adaption of subpopulations
within the biofilm through phenotypic expression of unique gene sets, which can
ensure the survival of the population as a whole, but often at the expense of
individuals within the biofilm. This mechanism can be considered either
altruism of a subset of the population or simply adaptation to the gradients of
growth conditions present in the biofilms discussed in section 2 that result in
the expression of different phenotypes. There have been many proposed models
for phenotypic plasticity of a bacterial population but we will focus on two
prominent hypotheses demonstrated in Figure 8.2. These are persister cell
populations and the genetic diversity associated with the insurance hypothesis.
a) Persister cells
Clonal populations of
cells, grown either as a planktonic or biofilm culture, give rise to persistent
subpopulations of cells that resist killing by high concentrations of antimicrobials.
Persistence is not the same as resistance, as persister populations possess no
resistance mechanisms carried on transposable elements. They survive but do not
grow in the presence of the selective agent, and when regrown the persister
population recapitulates the killing curve of the original population when
challenged again with the same antimicrobial. These persister populations
typically represent about 0.1% of a logarithmic planktonic culture and up to about
10% of the initial population in a biofilm and they may therefore account for
the higher level of antimicrobial tolerance seen in biofilms. Although
persister cells were first reported in the 1940s the molecular mechanisms
responsible for their properties are still a subject of debate. Persister cells
are not produced in response to a challenge but preexist in the population and
can be selected from any homogenous population of cells for being a slow growing,
physiological distinct subpopulations of small cells capable of tolerance to
environmental stress. The mechanism by which persistence is manifested remains
a focus of many studies. Persistence in E.
coli has been mapped to the high persistence operon (hip), containing the hi-pA
gene that encodes a toxin and the hip-B
gene that encodes an antitoxin that functions as a DNA-binding protein that
both binds Hip-A and also autoregulates the expression of the hip operon itself. Homologues of the hip operon are found across many
bacterial genera, suggesting this is a common mechanism of resistance.
Interestingly, in E. coli and other
species redundant toxin–antitoxin genes have been identified, suggesting the possible
specialization of sets of genes to deal with specific stress factors. Recently
a toxin– antitoxin pair, yafQdinJ has
been shown to act on biofilm but not planktonic populations to protect them against
very specific antimicrobials, which may provide a possible rationale for the
redundancy seen in these systems.
b) Subpopulations of cells— the ‘insurance hypothesis’
Biofilms are made up of
cells that have adapted to a wide range of physiological states associated with
the nutritional gradients within the biofilm, and hence even if the biofilm is
initiated from a clonal population it will display heterogeneity at both the
phenotypic and genotypic level. This diversity is enhanced when the biofilm is
placed under antimicrobial stress. Persister cell populations, discussed above,
represent only one adaptive state that contributes to the increased tolerance
of biofilms to antimicrobials. The combination of diverse populations found in
the biofilm that contribute to tolerance has been referred to as the insurance hypothesis, where diversity is
increased in an attempt to ensure a population will survive the stress of
antimicrobial challenge or increased metal or toxin concentrations in nature.
This is an area of immense interest and a field of study that is just in its
infancy. At this point numerous variants, separated on morphological criteria
from challenged populations, have been identified, but only now with the advent
of new-generation sequencing can these populations be screened for possible
mutations that lead to their tolerant phenotype. This is an area worth keeping
an eye on in the future.
Related Topics
