Monoaminopropyl analogues
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Antihistamines
Antihistamines -> H1-antagonists with classical structure - > Monoaminopropyl analogues -> Unsaturated analogues -> Triprolidine (Actidil) - Synthesis and Drug Profile
SYNTHESIS AND DRUG PROFILE
H1-antagonists with classical structure
Monoaminopropyl analogues
i. Unsaturated analogues
1. Triprolidine (Actidil)

Synthesis
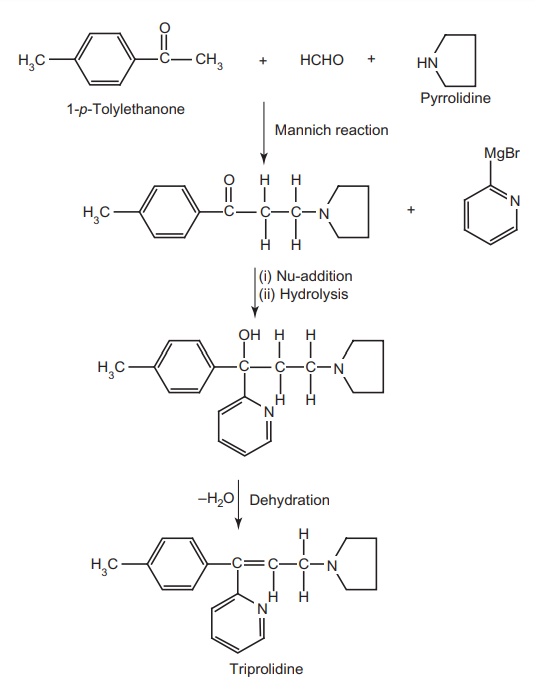
Properties and uses: Triprolidine hydrochloride is a white
crystalline powder, practically insoluble in ether, soluble in water and in
ethanol. The activity is mainly confined to the geometric isomer in which the
pyrrolidino-methyl group is trans to
the 2-pyridyl group. Pharmacological studies confirm the high activity of
triprolidine and the superiority of (E) over corresponding (Z) isomers as H1-antagonists.
In guinea pig ileum sites, the affinity of triprolidine (E) for H1-receptors
was more than 1000 times the affinity of its (Z) partner.
Assay: Dissolve the sample in a mixture of anhydrous acetic acid and
acetic anhydride and titrate against 0.1 M perchloric acid using crystal violet solution as
indicator.
Dose: Usual dose is 5–7.5 mg per day.
Dosage forms: Triprolidine HCl tablets I.P., Triprolidine tablets B.P.
Related Topics
