Neutral Carbon Nucleophiles
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Carbon-Carbon Bond Formation Between Carbon Nucleophiles and Carbon Electrophiles
Other types of carbon nucleophiles such as uncharged enol derivatives and other π -bonded systems are only weakly nucleophilic and consequently unreactive toward normal carbon electrophiles like carbonyl compounds or alkyl halides or sulfonates.
NEUTRAL CARBON NUCLEOPHILES
Other
types of carbon nucleophiles such as uncharged enol derivatives and other π -bonded systems are only weakly
nucleophilic and consequently unreactive toward normal carbon electrophiles
like carbonyl compounds or alkyl halides or sulfonates. For them to be used
effectively as nucleophiles, strong electrophiles must be used to match this
reduced nucleophilicity. This can be accomplished by increasing the reactivity
of a normal electrophile. Typically this is done by treating an electrophile
with a Lewis acid. Coordination of lone pairs of electrons with the Lewis acid
increases the electrophilicity markedly.
Examples
are
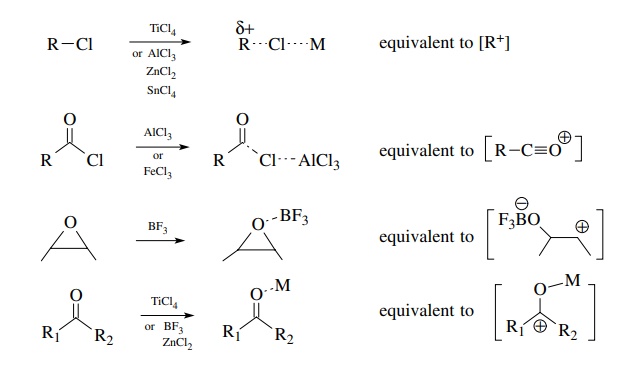
A
variety of Lewis acids can be used. Among the more commonly used ones are AlCl3,
TiCl4, SnCl4, ZnCl2, BF3, and
TMSOTf (trimethylsilyl triflate). The choice of Lewis acid is often critical to
the success of the reaction and is usually made by referring to similar
transformations that have been successfully reported in the literature. Often
it is not possible to rationalize why one Lewis acid works and another one does
not, so the initial choice of catalyst is normally made by literature
precedent. With the arsenal of Lewis acids available a suitable catalyst can
usually be found.
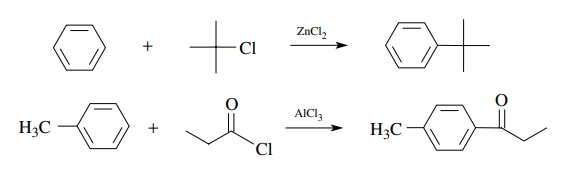
By
this strategy, reactive carbon electrophiles can be generated for successful
reaction with a variety of weak carbon nucleophiles. More important examples
include the Friedel–Crafts reaction in which aromatic compounds (nucleophiles)
react with alkyl and acyl halides (electrophiles in the presence of Lewis
acids).
The
Mukaiyama reaction is a versatile crossed-aldol reaction that uses a silyl enol
ether of an aldehyde, ketone, or ester as the carbon nucleophile and an
aldehyde or ketone activated by a Lewis acid as the carbon electrophile. The
product is a β-hydroxy carbonyl
compound typical of an aldol condensation. The advantages to this approach are
that it is carried out under acidic conditions and elimination does not usually
occur.
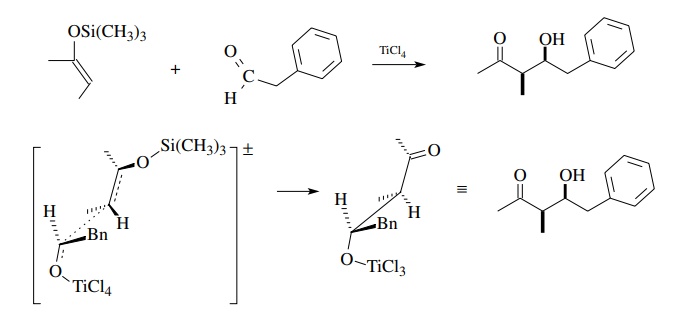
The
transition state is thought to be an open structure. Assuming that a particular
silyl enol ether geometry is used, the substituents will tend to occupy
opposite faces of the transition state and thus give a particular diastereomer
(syn–anti) preferentially. Because of the open transition state geometry, the
diastereoselec-tivity is not high.
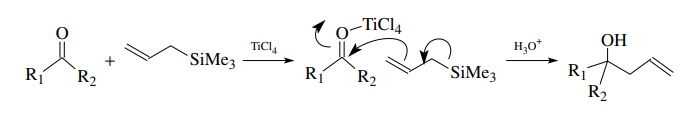
The
reaction of allyl silanes with aldehydes and ketones activated as
elec-trophiles by Lewis acids is a very useful method for preparing homoallylic
alcohols. Since allyl silanes are only modestly nucleophilic, strong
electrophiles are needed to ensure a good reactivity match.
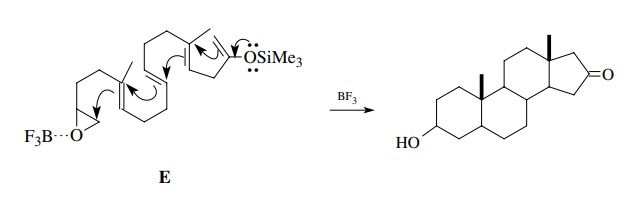
The
same considerations apply to intramolecular versions. For example, although
epoxide E is a stable compound,
treatment with a Lewis acid activates the epoxide as an electrophile and
cyclization with the olefinic π
system occurs to give the steroid ring system. Note that the silyl enol ether
group of E functions as the
terminating group. This polyene cyclization is similar to the way in which
steroids are biosynthesized. The cyclization is triggered by the generation of
an electrophile sufficiently strong to react intramolecularly with the weakly
nucleophilic π bond.
Related Topics
