Nuclear Magnetic Resonance
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Structure Determination of Organic Compounds
The speed with which NMR spectroscopy has been incorporated into scientific inquiry is truly amazing. The first commercial spectrometers became available in the 1950s.
NUCLEAR MAGNETIC RESONANCE
The
speed with which NMR spectroscopy has been incorporated into scientific inquiry
is truly amazing. The first commercial spectrometers became available in the
1950s. By the middle 1980s whole bodies could be placed in the probes of NMR
spectrometers (magnetic resonance imaging) and the structures of body parts
could be determined in exquisite detail. Today structures of proteins and other
macromolecules in solution or in the solid state are determined routinely. What
was unthinkable in the 1960s is routinely practiced today even by
under-graduates! The power of the method and the structural detail it provides
have no doubt fueled its rapid development.
Nuclear
magnetic resonance spectroscopy is possible due to the absorption of energy at
particular frequencies by atomic nuclei when they are placed in a magnetic
field. Most atomic nuclei are characterized by a property termed spin, and this gives rise to a magnetic
moment associated with that nucleus. The magnitude
of the magnetic moment of the nucleus, which is also quantized by the spin
quantum number, is characteristic of that nucleus. Nuclei such as 12C,
16O, and 32S have nuclear magnetic moments of zero. Other
nuclei such as 1H, 11B, 13C, 15N, 17O,
17O, 19F, and 31P have finite magnetic moments
and spin quantum numbers of I = 1/2 and are most useful in NMR
measurements. Still other nuclei such as 2D and 14N have
finite magnetic moments but spin numbers I
> 1/2 and are much more difficult to deal with, although today’s NMR
instruments handle these elements routinely as well.
Fortunately
for organic chemists, hydrogen and carbon are the most common nuclei found in
organic compounds, and the ability to probe these nuclei by NMR is invaluable
for organic structure determination. Since proton magnetic resonance (PMR) is
the most common type, the behavior of 1H nuclei in magnetic fields
will serve as a model for other nuclei which have spin quantum numbers I = 1/2 and thus behave similarly (13C, 19F, etc.).
The
proton has a nuclear magnetic moment (denoted as a vector quantity) which under
normal circumstances can adopt any spatial orientation. Since this magnetic
moment is a nuclear property, each hydrogen in a molecule has an identical
nuclear magnetic moment. When placed in a strong magnetic field, the magnetic
moment of the nucleus interacts with the magnetic field. The strength of the
interaction depends on the strength of the applied field (H0) and the
nuclear magnetic moment characterized by the magnetogyric ratio γ (the same for all hydrogens but
different for other nuclei).
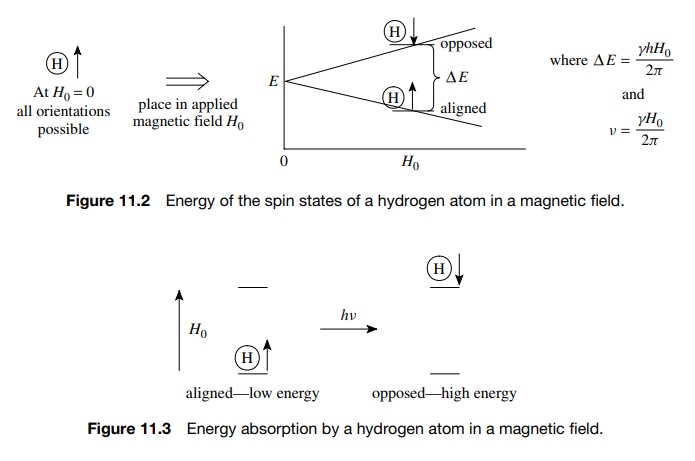
In
a strong magnetic field the nuclear magnetic moment is no longer free to adopt
just any orientation. Instead the spin quantum number of I = 12
for the hydrogen nucleus results in only two allowed orientations (2I + 1) of the nuclear moment relative
to the direction of the applied field — either aligned with H0 (lower energy) or opposed
to it (higher energy.) The difference in energy (ΔE) between the two states is given by ΔE = γ
hH0/2π and is dependent on the cross product
of the strength of the applied field H0
and the magnetic moment of the hydrogen (γ
). Since γ is the same for all
hydrogen nuclei, the energy difference between the two allowed orientations is
proportional only to the strength of the applied field (Figure 11.2).
If
the magnetic field H0 is
fixed and held very constant, then the energy gap between the two spin states
of the hydrogen nuclei will remain constant.
Irradiation
of the system with radiation of the appropriate frequency ( ΔE= hν) will cause the energy to be adsorbed
and the spin of the nucleus will flip from the low-energy state (aligned) to
the higher energy state (opposed). It is this absorption of energy which is
used to probe the structural features of the molecule (Figure 11.3).
Now since the magnetic moment of a nucleus (γ ) is an atomic property, for a given magnetic field H0, all hydrogens should absorb energy at the same frequency. However, examination of a molecule such as 1,2,2-trichloropropane (see Fig. 11.4) reveals that the two different types of hydrogens (H1 and H3) absorb at two different frequencies (ν1 and ν3) (Figure 11.4).
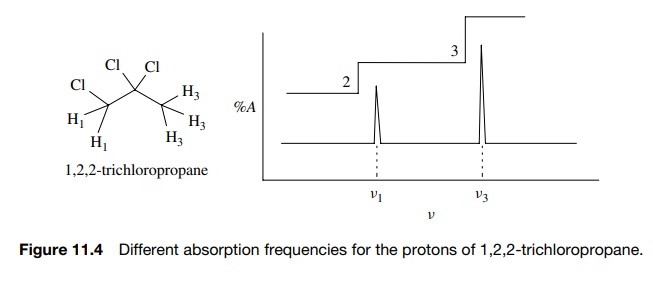
Since
the applied field H0 is
constant and all hydrogen nuclei have the same magnetic moment γ , the fact that H1 and H3
absorb at two different frequencies requires that the magnetic field that is
actually experienced by each set of nuclei (Heff)
is different. Stated differently, even though a constant magnetic field H0 is applied to the sample,
each type of hydrogen H1 and H3 experiences a unique
magnetic field Heff (where
Heff ≠ H0)
and consequently absorbs energy at a unique frequency ν1 and ν3.
Thus the different types of protons are distinguished by different frequencies
at which they absorb energy. Furthermore, integration of the absorption
intensities of the two signals gives a 3 : 2 ratio which corresponds to the
smallest whole-number ratio of each type of proton present. (The integrated
area of the peak is given by the step height on the integration curve.) Thus 1H
NMR is able to distinguish different types of protons in a molecule and tell
how many there are (at least by ratio).
Related Topics
