Physical Evaluation
| Home | | Pharmacognosy |Chapter: Pharmacognosy and Phytochemistry : Evaluation of Crude Drugs
In crude plant evaluation, physical methods are often used to determine the solubility, specific gravity, optical rotation, viscosity, refractive index, melting point, water content, degree of fibre elasticity, and other physical characteristics of the herb material.
PHYSICAL EVALUATION
In crude plant evaluation, physical methods are often used
to determine the solubility, specific gravity, optical rotation, viscosity,
refractive index, melting point, water content, degree of fibre elasticity, and
other physical characteristics of the herb material.
Solubility
Drugs specific behaviours towards solvents are taken into
consideration. This is useful for the examination of many oils, oleoresins,
etc. Few examples are the solubility of colophony in light petroleum, the
solubility of balsam of Peru in solution of chloral hydrate, the solubility of
castor oil in half its volume of light petroleum and the turbidity produced
with two volumes of the solvent; the solubility of balsam of Peru in an equal
volume of alcohol, 90%, and the production of a turbidity with a larger volume;
castor oil is soluble only in three volumes of 90% alcohol, while the
adulterated form it shows good solubility in alcohol. Alkaloidal bases are
soluble in organic solvents and alkaloidal salts are soluble in polar solvents.
Optical Rotation
Anisotropic crystalline solids and samples containing an
excess of one enantiomer of a chiral molecule can rotate the orientation of
plane-polarized light. Such substances are said to be optically active, and
this property is known as optical rotation. The enantiomer that rotates light
to the right, or clockwise when viewing in the direction of light propagation,
is called the dextrorotatory (d) or (+) enantiomer, and the enantiomer that
rotates light to the left, or counterclockwise, is called the levorotatory (l)
or ({) enantiomer. Few examples of drugs with this property are
eucalyptus oil (0° to +10°), honey (+3° to {15°),
Che-nopodium oil ({30° to {80°), etc.
Refractive Index
Refractive index is defined as the property of a material
that changes the speed of light, computed as the ratio of the speed of light in
a vacuum to the speed of light through the material. When light travels at an
angle between two different materials, their refractive indices determine the
angle of transmission refraction of the light beam. In general, the refractive
index varies based on the frequency of the light as well; thus, different
colours of light travel at different speeds. High intensities can also change
the refractive index. This could be used as a parameter in evaluating the
herbal drugs; for example castor oil 1.4758 to 1.527, clove oil 1.527 to 1.535,
etc.
Specific Gravity
It is also known as relative density. The ratio of the mass
of a solid or liquid to the mass of an equal volume of distilled water at 4°C
(39°F) or of a gas to an equal volume of air or hydrogen under prescribed
conditions of temperature and pressure. Some examples of specific gravity of
drugs are cottonseed oil 0.88–0.93, coconut oil 0.925, castor oil 0.95, etc.
Viscosity
Viscosity is the resistance of a fluid to flow. This
resistance acts against the motion of any solid object through the fluid and
also against motion of the fluid itself past stationary obstacles. Viscosity of
a liquid is constant at a given tempera-ture and is an index of its
composition. Viscosity also acts internally on the fluid between slower- and
faster-moving adjacent layers. Since it is constant at a given temperature, it
is used as an evaluation parameter; for example, pyroxylin kinematic viscosity,
1100–2450 centistokes.
Melting Point
The melting point of a solid is the temperature at which it
changes state from solid to liquid. Plant constituents have very sharp and
constant melting points. As far as crude drugs are concerned, melting point
range has been fixed due to mixed chemicals. The following drugs could be
evaluated using this parameter; for example, beeswax 62–65°C, wool fat 34–44°C,
agar melts at 85°C, etc.
Moisture Content
The moisture content of a drug will be responsible for
decomposition of crude drugs either producing chemical change or microbial
growth. So the moisture content of a drug should be determined and controlled.
The moisture content is determined by heating a drug at 105°C in an oven to a
constant weight. Following are the examples of two crude drugs with their
moisture content limit: the moisture content of Digitalis and Ergot should not
be more than 5% w/w and 8% w/w, respectively.
Ultraviolet Light
Certain drugs fluoresce when the cut surface or the powder
is exposed to ultraviolet radiation, and it is useful in the identification of
those drugs. Some pieces of rhapontic, Indian, and Chinese rhubarb are very
difficult to distinguish, and it is very difficult in powdered form, but
examination in ultraviolet light gives such marked differences in fluorescence
that the varieties can be easily distinguished from each other.
Ash Values
The determination of ash is useful for detecting low-grade
products, exhausted drugs, and excess of sandy or earthy matter. Different
types of ash values are used in detection of crude drugs like, total ash,
acid-insoluble ash, water-soluble ash, and sulphated ash.
Total ash is useful in detecting the crude drugs that are
mixed with various mineral substances like sand, soil, calcium oxalate, chalk
powder, or other drugs with differ-ent inorganic contents to improve their
appearance, as is done with nutmegs and ginger. The maximum temperature used
for total ash should be not more than 450°C because alkali chlorides that may
be volatile in higher temperatures would be lost.
Acid-insoluble ash means the ash insoluble in dilute
hydrochloric acid. It is often of more value than the total ash. The majority
of crude drugs contain calcium oxalate, and the quantity of calcium oxalate
varies very frequently. So total ash of a crude drug vary within wide limits
for specimens of genuine drug, for example, rhubarb, total ash range from 8 to
40%. In this case, the total ash is useless to detect earthy matter adherent to
such a drug. So acid-insoluble ash would be preferable for rhubarb. The calcium
oxide or carbonate, yielded by the incinerated oxalate, will be soluble in
hydrochloric acid when the ash is treated with hydrochloric acid; the remaining
ash is weighed, which is known as the acid-insoluble ash. By this we can detect
the presence of excessive earthy matter, which is likely to occur with roots
and rhizomes and with leaves which are densely pubescent, like those of
foxglove, clothed with abundant trichomes secreting resin, as in henbane, and
tend to retain earth matter splashed on to them during heavy rainstorms.
The water-soluble ash is used to detect the presence of
material exhausted by water. Sulphated ash is done by addition of sulphuric
acid in order to get sulphate salts, and the percentage ash is calculated with
reference to the air-dried drug. The temperature used for this is above 600°C.
The total ash and acid-insoluble ash values of Guduchi are not more than 16 and
3%, respectively. The total ash value and water-soluble ash values of ginger
are 6 and 1.7%, respectively.
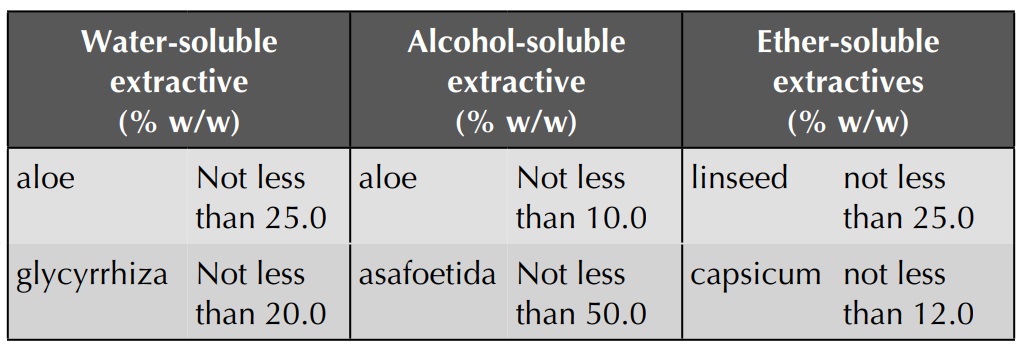
Extractive Values
The extracts obtained by exhausting crude drugs with
different solvents are approximate measures of their chemical constituents.
Various solvents are used according to the type of the constituents to be
analysed. Water-soluble extractive is used for crude drugs containing
water-soluble constituents like glycosides, tannins, mucilage, etc.;
alcohol-soluble extractive is used for crude drugs containing tannins,
glycosides, resins, etc.; and ether-soluble extractives are used for drugs
containing volatile constituents and fats.
Extractive Values of Some Crude Drugs
Foreign Organic Matters
The parts of the organ or organs other than those parts of
drugs mentioned in the definition and description of the drug are known as
foreign organic matters. They may be insect, moulds, earthy material, animal
excreta, etc. Each and every vegetable drug has their own limits. Few examples
of such limits are: garlic should not contain more than 2%, saffron should not
contain more than 2%, satavari should not contain more than 1%, etc.
Related Topics
