Physical Properties of Enantiomers
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Stereochemical and Conformational Isomerism
If both enantiomers are present in a solid sample, the melting point and the solubility of the solid mixture are often found to be different than those of the pure enantiomers.
PHYSICAL PROPERTIES OF
ENANTIOMERS
If
both enantiomers are present in a solid sample, the melting point and the
solubility of the solid mixture are often found to be different than those of
the pure enantiomers. This is due to the fact that the solid-state interaction
of two R enantiomers or two S enantiomers is often different than the
solid-state interaction of an R and an S enantiomer. (In fact, these
interactions are diastereomeric.) The result is that three different scenarios
are possible when a racemic mixture is crystallized from solution:
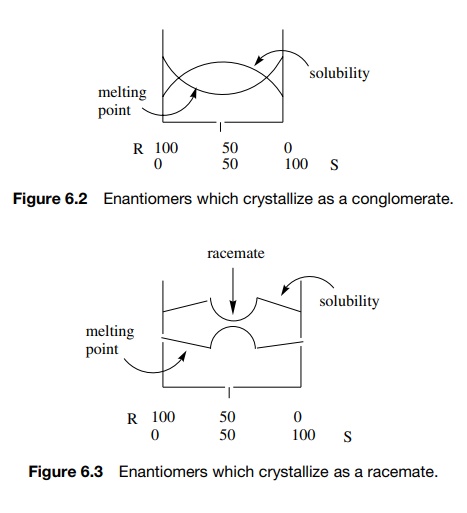
1. If an enantiomer has a greater affinity for molecules of
like configuration, then two sets of crystals will be formed, one set composed
only of the (+) form and the other
composed only of the (−)
form. This racemic mixture is called a conglomerate because it is a mixture of
two different types of crystals. Moreover it behaves as a typical mixture —the
melting point is lower than the pure enantiomeric components and the solubility
is higher (Figure 6.2). This is a relatively rare situation.
2. If an enantiomer has a greater affinity for molecules of opposite configu-ration, then crystals are produced which contain equal numbers of the (+) and (−) forms.
The solid
compound which has properties different than either pure enantiomer and exists
only in the solid state is called a race-mate or a racemic compound or a
racemic mixture. A racemate is often higher melting and less soluble than a
pure enantiomer and behaves as a mixture in the presence of either pure
enantiomer (Figure 6.3). This is the most common situation and allows an
unequal mixture of enantiomers to be purified. Upon crystallization, the
racemate will precipitate first, leaving behind the enantiomer in excess.
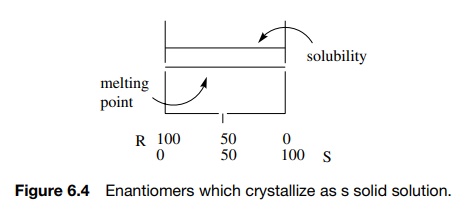
3. If one enantiomer has similar affinity for molecules of
either configuration, then the enantiomers are randomly distributed in the
crystal and the solid is a “racemic solid solution” or mixed crystal. Such
solids are identical with either enantiomer (Figure 6.4).
Related Topics
