Polymer molecular weight and weight distribution
| Home | | Pharmaceutical Drugs and Dosage | | Pharmaceutical Industrial Management |Chapter: Pharmaceutical Drugs and Dosage: Pharmaceutical polymers
Both synthetic and natural polymers exist in a range of sizes, defined by the number of monomeric units or their molecular weights.
Polymer molecular
weight and weight distribution
Both
synthetic and natural polymers exist in a range of sizes, defined by the number
of monomeric units or their molecular weights. The polymeriza-tion process
produces polymers of different sizes. Thus, any given batch or quantity of a
polymer is a mixture of polymers of different sizes. The nominal (or labeled)
molecular weight of a polymer is an average molecu-lar weight, which is
inferred by the bulk property of the polymer such as chemical analysis, osmotic
pressure, or light scattering. When deter-mined by chemical analysis or osmotic
pressure measurement, the reported molecular weight is the number average molecular weight, Mn, since these analytical tools are sensitive to the
number of polymer chains of differ-ent sizes. Thus, for a mixture containing n1, n2, n3,
… moles of polymers with molecular weights M1,
M 2, M3, …, respectively, the number average molecular weight
is defined by:
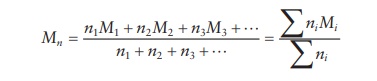
Thus,
the number average molecular weight is the arithmetic mean of the molecular
weight of all the polymer chains in the sample.
On
the other hand, measurement techniques such as light scattering pro-duce a
response that depends on the molecular weight of the polymer chain. Thus,
larger molecules produce greater light scattering. The molecular weight of the
polymer chain or species of each size carries greater weight-age in generating
the measured response. Thus, the molecular weight is weighted in the inference
of molecular weight by using such techniques. Such a molecular weight is
defined as the weight average molecular weight, Mw. The weight average molecular weight for the same
sample would be defined as:
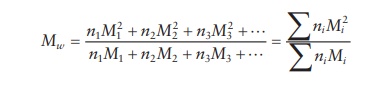
The
weight average molecular weight is generally greater than the num-ber average
molecular weight. Thus, the average polymer molecular weight measured by light
scattering is greater than the polymer molecular weight obtained by osmotic
pressure measurement.
Related Topics
