Definitions
| Home | | Pharmaceutical Microbiology | | Pharmaceutical Microbiology |Chapter: Pharmaceutical Microbiology : Principles Of Good Manufacturing Practice
Several terms used in industrial and hospital production must be defined to enable the reader to follow this article.
DEFINITIONS
Several terms used in industrial and
hospital production must be defined to enable the reader to follow this article.
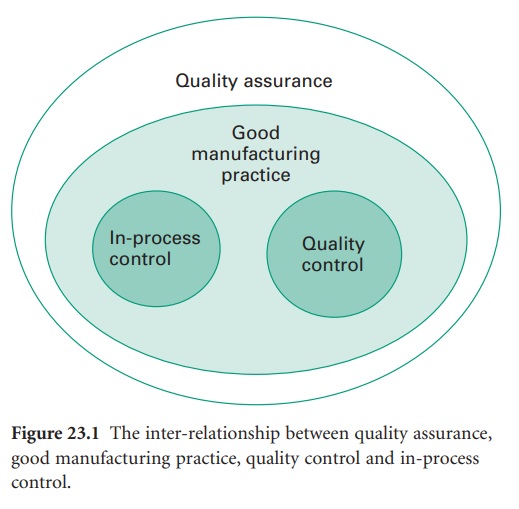
The inter-relationship between quality assurance (QA), GMP, quality control
(QC) and in-process control is shown in Figure 23.1.
The UK Orange Guide (The Rules and Guidance for Pharmaceutical Manufacturers and
Distributors, 2007) emphasizes the fundamental point:
Quality assurance is particularly important, and this type of manufacture
must strictly follow carefully established and validated methods of preparation
and procedure. Sole reliance for sterility or other quality aspects must not be
placed on any terminal sterilization process or finished product test.
The difficulty in demonstrating quality
is that the tests carried out are designed to show the absence of quality. For
example, the test for sterility involves taking samples and testing for
microorganisms. If 20 samples are tested, 3.4% of the batch needs to be
contaminated to have a 50% chance of detecting that contamination. That level
of contamination represents gross failure of GMP and problems would normally be
detected by environmental monitoring. Indeed, it is not unknown for a batch to
pass a sterility test but be rejected due to problems detected during
environmental monitoring (section 5.2). Therefore it is important that a
product be manufactured in a suitable environment by a procedure that minimizes
the possibility of contamination occurring. At the end of this process the
tests can be performed as an additional measure
of quality.
A) Quality
There are many definitions of quality (see Sharp, 2000). For the purpose of
pharmaceutical products the term quality is
usually taken to mean fitness for purpose.
Not only must the product have the desired therapeutic properties, it must also
be safe for administration by the route intended. Sharp (2000, 2001) discussed
several meanings of quality but summarizes it as follows:
in a nutshell, it is fit to be given to a patient in the confidence that
it will have the desired effects and not damage him or her, in any way, through
faults in manufacture.
B) Manufacture
Manufacture is the complete cycle of production of a medical product.
This cycle includes the acquisition of all raw materials, their processing into
a final product, and subsequent packaging and distribution.
C) Quality Assurance (QA)
Quality assurance is a wide ranging concept covering all matters which
individually or collectively influence the quality of a product. It is the
total sum of the procedures needed to ensure the fitness of a pharmaceutical
product for its intended use. QA incorporates GMP plus other factors.
D) Good Manufacturing Practice (GMP)
GMP is that part of QA which is aimed at ensuring the product is
consistently manufactured to a quality appropriate for its intended use and to
meet the requirements of the regulatory authorities. GMP requires that: (1) the
manufacturing process is fully defined before it begins; and (2) the necessary
facilities are provided. In practice, this means that:
·
Personnel must be adequately trained..
·
Suitable premises and equipment must be
employed.
·
Correct materials must be used.
·
Approved procedures must be adopted.
·
Suitable storage and transport
facilities must be available.
·
Appropriate records must be made.
The reasons for GMP are (Sharp, 2001):
·
the poor chance of the patient
detecting that anything is wrong
·
the weakness of product testing
because:
·
we can only test samples
·
we cannot test for everything
·
the dangers to patients of even only a
small number of defective or wrongly labelled items in a batch (and it is very
difficult to detect a small number of defectives).
It is about getting things right all along the line.
E) Quality Control (QC)
QC is that part of GMP concerned with
sampling, specifications and testing, as well as the organization,
documentation and release procedures which ensure that the necessary and relevant
tests are carried out, and that materials are not released for use, nor
products released for sale or supply, until their quality has been judged
satisfactory. For sterile products QC includes testing for sterility and
pyrogens. The Rules and Guidance for Pharmaceutical Manufacturers and
Distributors (2007) states that QC is not confined to
laboratory operations, but must be involved in all decisions which may affect
the quality of the product. The independence of QC from production is
considered fundamental to the satisfactory operation of QC.
F) In-Process Control
This comprises any test on a product, the environment or the equipment
that is used during the manufacturing process. An example of this is testing
that an autoclave is functioning correctly (Gardner & Peel, 1998).
G) Validation
A documented programme that provides a high degree of assurance that a
specific process, method or system will consistently produce a result meeting
predetermined acceptance criteria.
Related Topics
