Protein and peptide drug delivery
| Home | | Pharmaceutical Drugs and Dosage | | Pharmaceutical Industrial Management |Chapter: Pharmaceutical Drugs and Dosage: Protein and peptide drug delivery
The use of therapeutic proteins to replace or supplement endogenous protein molecules has been a long established treatment for diseases such as diabetes, growth hormone deficiency, and hemophilia.
Protein and
peptide drug delivery
Introduction
The
use of therapeutic proteins to replace or supplement endogenous protein
molecules has been a long established treatment for diseases such as diabetes,
growth hormone deficiency, and hemophilia. The use of proteins and peptides as
pharmaceutical products has increased significantly in recent years with the
commercialization of monoclonal antibody (mAb)-based therapeutics such as
immune-oncology agents Opdivo® and Yervoy®, and the antibody– drug conjugates
(ADCs) such as Kadcyla® and Adcentris®.
Protein
and peptide drugs are either natural in origin or synthetically produced using
recombinant DNA technology or from transgenic animals. Recombinant DNA
technology has allowed the large-scale production and biological
characterization of several therapeutic proteins, including gran-ulocyte
macrophage colony-stimulating factor (GM-CSF), erythropoietin (EPO),
interleukins, insulin-like growth factor-1 (IGF-1), human factors VIII and IX
(involved in blood coagulation and useful for hemophilia), mAbs, and tissue
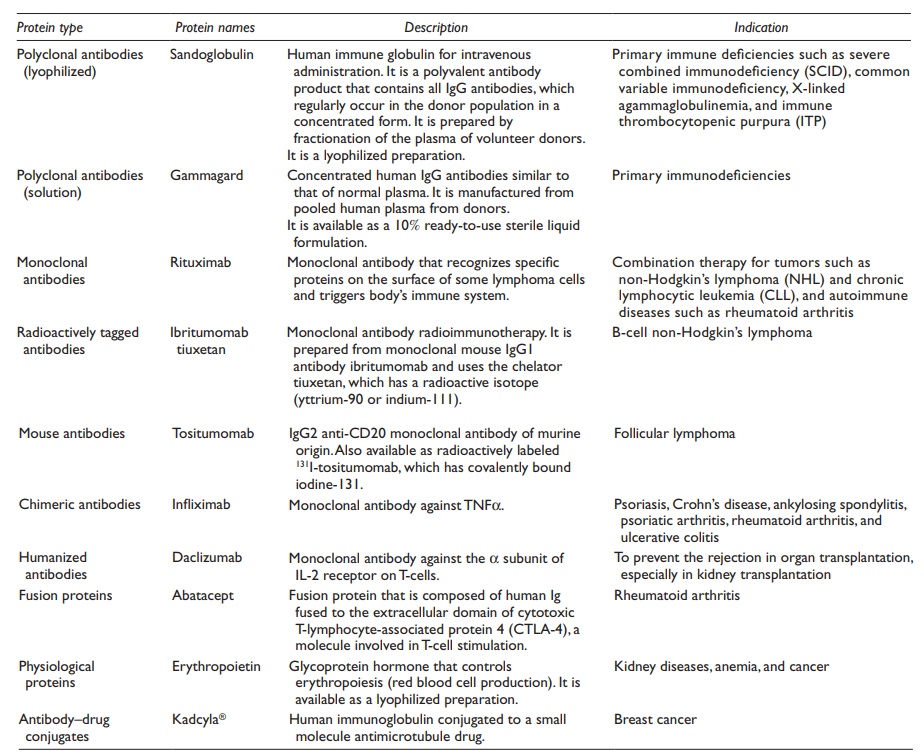
plasminogen activator (t-PA). Table 25.1 lists
some of the FDA-approved marketed products of therapeutic proteins.
Table 25.1 List of some commercial products of therapeutic proteins
The
physical and chemical instabilities of proteins and peptides, arising from
their large molecular weight and complex structure, pose many chal-lenges for
pharmaceutical formulation development. Clinical applications of protein drugs
are limited by their inadequate concentration in blood, poor oral
bioavailability, high manufacturing cost, chemical or biologi-cal instability,
and/or rapid hepatic metabolism. In addition, most protein drugs do not
efficiently pass through biological membranes and enter their target cells.
These limitations lead to their high dose and/or need for fre-quent
administration, which can cause undesirable side effects. Also, pro-teins can
elicit host immune response following repeated use due to the development of
neutralizing antibodies or hypersensitivity reactions.
Proteins
and peptides are rapidly degraded in the gastrointestinal tract due to the
harsh pH and enzymatic environment, resulting in poor oral bioavail-ability.
Therefore, proteins are primarily administered parenterally by intrave-nous
(IV), subcutaneous (SC), and/or intramuscular (IM) injection. Thus, the
development of a protein formulation is primarily focused on a sterile
solu-tion or a sterile, lyophilized powder for reconstitution prior to
administration.
Related Topics
