Reactions of Glycolysis
| Home | | Biochemistry |Chapter: Biochemistry : Introduction to Metabolism and Glycolysis
The conversion of glucose to pyruvate occurs in two stages. The first five reactions of glycolysis correspond to an energy–investment phase in which the phosphorylated forms of intermediates are synthesized at the expense of ATP.
REACTIONS OF GLYCOLYSIS
The conversion of
glucose to pyruvate occurs in two stages (Figure 8.11). The first five
reactions of glycolysis correspond to an energy–investment phase in which the
phosphorylated forms of intermediates are synthesized at the expense of ATP.
The subsequent reactions of glycolysis constitute an energy–generation phase in
which a net of two molecules of ATP are formed by substrate-level
phosphorylation per glucose molecule metabolized.
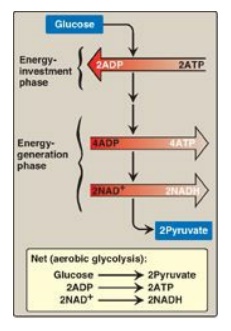
Figure 8.11 Two phases of aerobic glycolysis. NAD(H) = nicotinamide adenine dinucleotide.
A. Phosphorylation of glucose
Phosphorylated sugar
molecules do not readily penetrate cell membranes because there are no specific
transmembrane carriers for these compounds and because they are too polar to
diffuse through the lipid core of membranes. The irreversible phosphorylation
of glucose (Figure 8.12), therefore, effectively traps the sugar as cytosolic
glucose 6-phosphate, thereby committing it to further metabolism in the cell.
Mammals have four (I–IV) isozymes of the enzyme hexokinase that catalyze the
phosphorylation of glucose to glucose 6-phosphate.
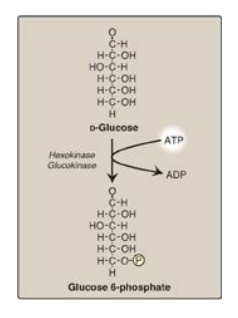
Figure 8.12 Energy-investment phase: phosphorylation of glucose. [Note: Kinases utilize ATP complexed with a divalent metal ion, most typically Mg2+.]
1. Hexokinases I–III: In most tissues, phosphorylation of glucose is catalyzed by one of these isozymes of hexokinase, which is one of three regulatory enzymes of glycolysis (see also phosphofructokinase and pyruvate kinase). These isozymes have broad substrate specificity and are able to phosphorylate several hexoses in addition to glucose. They are inhibited by the reaction product, glucose 6-phosphate, which accumulates when further metabolism of this hexose phosphate is reduced. Hexokinases I-III have a low Michaelis constant (Km) (and, therefore, a high affinity;) for glucose. This permits the efficient phosphorylation and subsequent metabolism of glucose even when tissue concentrations of glucose are low (Figure 8.13). These isozymes, however, have a low maximal velocity ([V max]) for glucose and, therefore, do not sequester (trap) cellular phosphate in the form of phosphorylated hexoses, or phosphorylate more sugars than the cell can use.
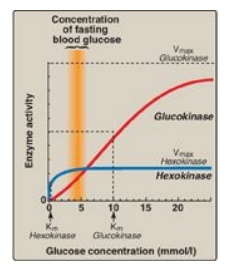
Figure 8.13 Effect of glucose concentration on the rate of phosphorylation catalyzed by hexokinase and glucokinase. Km = Michaelis constant; V max = maximal velocity.
2. Hexokinase IV (or, glucokinase): In liver parenchymal cells and b
cells of the pancreas, glucokinase (the hexokinase IV isozyme) is the
predominant enzyme responsible for the phosphorylation of glucose. In β cells,
glucokinase functions as a glucose sensor, determining the threshold for
insulin secretion. [Note: Hexokinase IV also serves as a glucose sensor in
neurons of the hypothalamus, playing a key role in the adrenergic response to
hypoglycemia.] In the liver, the enzyme facilitates glucose phosphorylation
during hyperglycemia. Despite the popular but misleading name glucokinase, the
sugar specificity of the enzyme is similar to that of other hexokinase
isozymes.
a. Kinetics: Glucokinase differs from hexokinases I–III in
several important properties. For example, it has a much higher Km,
requiring a higher glucose concentration for half-saturation (see Figure 8.13).
Thus, glucokinase functions only when the intracellular concentration of
glucose in the hepatocyte is elevated such as during the brief period following
consumption of a carbohydrate-rich meal, when high levels of glucose are
delivered to the liver via the portal vein. Glucokinase has a high Vmax,
allowing the liver to effectively remove the flood of glucose delivered by the
portal blood. This prevents large amounts of glucose from entering the systemic
circulation following such a meal thereby minimizing hyperglycemia during the
absorptive period. [Note: GLUT-2 insures that blood glucose equilibrates
rapidly across the membrane of the hepatocyte.]
b. Regulation by fructose 6-phosphate and glucose: Glucokinase activity is not directly inhibited by glucose 6-phosphate as are the other hexokinases but, rather, is indirectly inhibited by fructose 6-phosphate (which is in equilibrium with glucose 6-phosphate, a product of glucokinase) and is indirectly stimulated by glucose (a substrate of glucokinase) via the following mechanism. Glucokinase regulatory protein (GKRP) in the liver regulates the activity of glucokinase through reversible binding. In the presence of fructose 6-phosphate, glucokinase is translocated into the nucleus and binds tightly to the regulatory protein, thereby rendering the enzyme inactive (Figure 8.14). When glucose levels in the blood (and also in the hepatocyte, as a result of GLUT-2) increase, glucokinase is released from the regulatory protein, and the enzyme reenters the cytosol where it phosphorylates glucose to glucose 6-phosphate. [Note: Fructose 1-phosphate inhibits formation of the glucokinase–GKRP complex.]
Glucokinase functions as a glucose sensor in the
maintenance of blood glucose homeostasis. Inactivating mutations of glucokinase
are the cause of a rare form of diabetes, maturity onset diabetes of the young
type 2 (MODY 2) that is characterized by impaired insulin secretion.
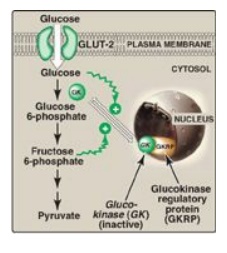
Figure 8.14 Regulation of
glucokinase activity by glucokinase regulatory protein. GLUT = glucose
transporter.
B. Isomerization of glucose 6-phosphate
The isomerization of
glucose 6-phosphate to fructose 6-phosphate is catalyzed by phosphoglucose
isomerase (Figure 8.15). The reaction is readily reversible and is not a
rate-limiting or regulated step.
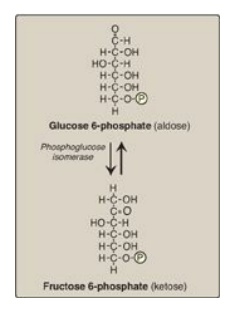
Figure 8.15 Aldose-ketose isomerization of glucose 6-phosphate to fructose 6-phosphate. P = phosphate.
C. Phosphorylation of fructose 6-phosphate
The irreversible
phosphorylation reaction catalyzed by phosphofructokinase-1 (PFK-1) is the most
important control point and the rate-limiting and committed step of glycolysis
(Figure 8.16) . PFK-1 is controlled by the available concentrations of the
substrates ATP and fructose 6-phosphate as well as by regulatory substances
described below.
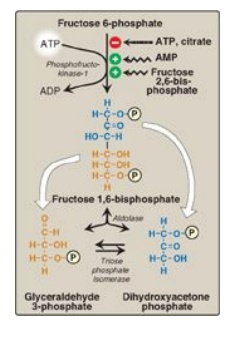
Figure 8.16 Energy-investment phase (continued): Conversion of fructose 6-phosphate to triose phosphates. P = phosphate; AMP = adenosine monophosphate.
1. Regulation by energy levels within the cell: PFK-1 is inhibited allosterically
by elevated levels of ATP, which act as an “energy-rich” signal indicating an
abundance of high-energy compounds. Elevated levels of citrate, an intermediate
in the TCA cycle, also inhibit PFK-1. [Note: Inhibition by citrate favors the
use of glucose for glycogen synthesis.] Conversely, PFK-1 is activated allosterically
by high concentrations of AMP, which signal that the cell’s energy stores are
depleted.
2. Regulation by fructose 2,6-bisphosphate: Fructose 2,6-bisphosphate is the most potent activator of PFK-1 (see Figure 8.16) and is able to activate the enzyme even when ATP levels are high. Fructose 2,6-bisphosphate is formed from fructose 6-phosphate by phosphofructokinase-2 (PFK-2), an enzyme different than PFK-1. PFK-2 is a bifunctional protein that has both the kinase activity that produces fructose 2,6-bisphosphate and the phosphatase activity that dephosphorylates fructose 2,6-bisphosphate back to fructose 6-phosphate. In the liver, the kinase domain is active if dephosphorylated and is inactive if phosphorylated (Figure 8.17). [Note: Fructose 2,6-bisphosphate is an inhibitor of fructose 1,6-bisphosphatase, an enzyme of gluconeogenesis. The reciprocal actions of fructose 2,6-bisphosphate on glycolysis (activation) and gluconeogenesis (inhibition) ensure that both pathways are not fully active at the same time, preventing a futile cycle in which glucose would be converted to pyruvate followed by resynthesis of glucose from pyruvate.]
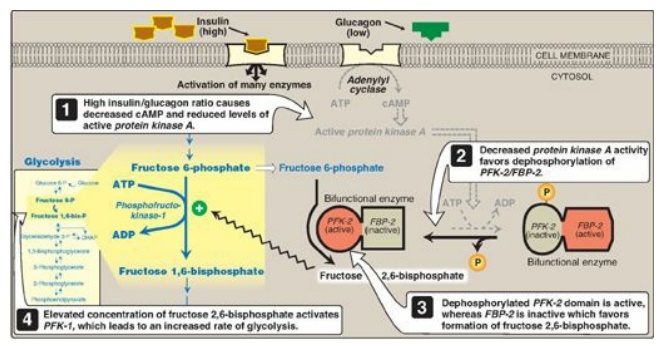
Figure 8.17 Effect of elevated insulin concentration on the intracellular concentration of fructose 2,6-bisphosphate in liver. PFK-2 = phosphofructokinase-2; FBP-2 = fructose 2,6-bisphosphatase; cAMP = cyclic AMP; P = phosphate.
a. During the well-fed state: Decreased levels of glucagon and
elevated levels of insulin, such as occur following a carbohydrate-rich meal,
cause an increase in fructose 2,6-bisphosphate and, thus, in the rate of
glycolysis in the liver (see Figure 8.17). Fructose 2,6-bisphosphate,
therefore, acts as an intracellular signal, indicating that glucose is
abundant.
b. During fasting: Elevated levels of glucagon and low levels of insulin, such as occur during fasting, decrease the intracellular concentration of hepatic fructose 2,6-bisphosphate. This results in inhibition of glycolysis and activation of gluconeogenesis.
Cleavage of fructose 1,6-bisphosphate
Aldolase cleaves fructose 1,6-bisphosphate to dihydroxyacetone phosphate and glyceraldehyde 3-phosphate (see Figure 8.16). The reaction is reversible and not regulated. [Note: Aldolase B, the isoform found primarily in the liver, also cleaves fructose 1-phosphate and functions in the metabolism of dietary fructose.]
E. Isomerization of dihydroxyacetone phosphate
Triose phosphate
isomerase interconverts dihydroxyacetone phosphate (DHAP) and glyceraldehyde
3-phosphate (see Figure 8.16). DHAP must be isomerized to glyceraldehyde
3-phosphate for further metabolism by the glycolytic pathway. This
isomerization results in the net production of two molecules of glyceraldehyde
3-phosphate from the cleavage products of fructose 1,6-bisphosphate. [Note:
DHAP is utilized in triacylglycerol synthesis.]
F. Oxidation of glyceraldehyde 3-phosphate
The conversion of
glyceraldehyde 3-phosphate to 1,3-bisphosphoglycerate (1,3-BPG) by
glyceraldehyde 3-phosphate dehydrogenase is the first oxidation-reduction
reaction of glycolysis (Figure 8.18). [Note: Because there is only a limited
amount of NAD+ in the cell, the NADH formed by this reaction must be
reoxidized to NAD+ for glycolysis to continue. Two major mechanisms
for oxidizing NADH are 1) the NADH-linked conversion of pyruvate to lactate
(anaerobic;) and 2) oxidation of NADH via the respiratory chain (aerobic;). The
latter requires the malate-aspartate and glycerol 3-phosphate substrate
shuttles.]
1. Synthesis of 1,3-bisphosphoglycerate: The oxidation of the aldehyde group
of glyceraldehyde 3-phosphate to a carboxyl group is coupled to the attachment
of Pi to the carboxyl group. The high-energy phosphate group at carbon 1 of
1,3-BPG conserves much of the free energy produced by the oxidation of
glyceraldehyde 3-phosphate. The energy of this high-energy phosphate drives the
synthesis of ATP in the next reaction of glycolysis.
2. Mechanism of arsenic poisoning: The toxicity of arsenic is due
primarily to the inhibition by trivalent arsenic (arsenite) of enzymes such as
the pyruvate dehydrogenase complex, which require lipoic acid as a coenzyme.
However, pentavalent arsenic (arsenate) can prevent net ATP and NADH production
by glycolysis without inhibiting the pathway itself. It does so by competing
with P i as a substrate for glyceraldehyde 3-phosphate dehydrogenase, forming a
complex that spontaneously hydrolyzes to form 3-phosphoglycerate (see Figure
8.18). By bypassing the synthesis of and phosphate transfer from 1,3-BPG, the
cell is deprived of energy usually obtained from the glycolytic pathway. [Note:
Arsenate also competes with Pi on the F1 domain of ATP synthase,
resulting in formation of ADP-arsenate that is rapidly hydrolyzed.]
3. Synthesis of 2,3-bisphosphoglycerate in red
blood cells: Some
of the 1,3-BPG is converted to 2,3-BPG by the action of bisphosphoglycerate
mutase (see Figure 8.18). 2,3-BPG, which is found in only trace amounts in most
cells, is present at high concentration in red blood cells (RBCs) and serves to
increase O2 delivery. 2,3-BPG is hydrolyzed by a phosphatase to
3-phosphoglycerate, which is also an intermediate in glycolysis (see Figure
8.18). In the RBC, glycolysis is modified by inclusion of these “shunt”
reactions.
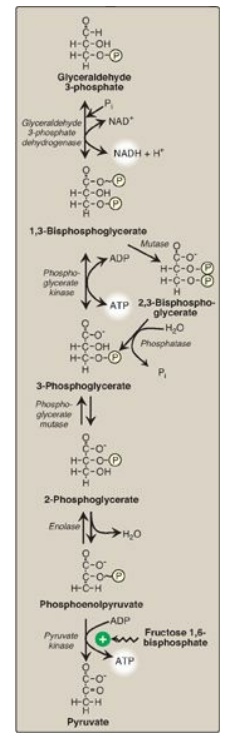
Figure 8.18 Energy-generating
phase: conversion of glyceraldehyde 3-phosphate to pyruvate. NAD(H) =
nicotinamide adenine dinucleotide P = phosphate; Pi = inorganic phosphate.
G. Synthesis of 3-phosphoglycerate, producing ATP
When 1,3-BPG is
converted to 3-phosphoglycerate, the high-energy phosphate group of 1,3-BPG is
used to synthesize ATP from ADP (see Figure 8.18). This reaction is catalyzed
by phosphoglycerate kinase, which, unlike most other kinases, is
physiologically reversible. Because two molecules of 1,3-BPG are formed from
each glucose molecule, this kinase reaction replaces the two ATP molecules
consumed by the earlier formation of glucose 6-phosphate and fructose
1,6-bisphosphate. [Note: This is an example of substrate-level phosphorylation,
in which the energy needed for the production of a high-energy phosphate comes
from a substrate rather than from the electron transport chain (see J. below).]
H. Shift of the phosphate group
The shift of the
phosphate group from carbon 3 to carbon 2 of phosphoglycerate by
phosphoglycerate mutase is freely reversible (see Figure 8.18).
I. Dehydration of 2-phosphoglycerate
The dehydration of
2-phosphoglycerate by enolase redistributes the energy within the substrate,
resulting in the formation of phosphoenolpyruvate (PEP), which contains a
high-energy enol phosphate (see Figure 8.18). The reaction is reversible
despite the high-energy nature of the product. [Note: Fluoride inhibits
enolase, and water fluoridation reduces lactate production by mouth bacteria,
decreasing dental caries.]
J. Formation of pyruvate, producing ATP
The conversion of PEP
to pyruvate is catalyzed by pyruvate kinase (PK), the third irreversible
reaction of glycolysis. The high-energy enol phosphate in PEP is used to
synthesize ATP from ADP and is another example of substrate-level
phosphorylation (see Figure 8.18).
1. Feedforward regulation: PK is activated by fructose
1,6-bisphosphate, the product of the phosphofructokinase-1 reaction. This
feedforward (instead of the more usual feedback) regulation has the effect of
linking the two kinase activities: increased phosphofructokinase activity
results in elevated levels of fructose 1,6-bisphosphate, which activates PK.
2. Covalent modulation of pyruvate kinase: Phosphorylation by a cAMP-dependent protein kinase leads to inactivation of the hepatic isozyme of PK (Figure 8.19). When blood glucose levels are low, elevated glucagon increases the intracellular level of cAMP, which causes the phosphorylation and inactivation of PK in the liver only. Therefore, PEP is unable to continue in glycolysis and, instead, enters the gluconeogenesis pathway. This, in part, explains the observed inhibition of hepatic glycolysis and stimulation of gluconeogenesis by glucagon. Dephosphorylation of PK by a phosphatase results in reactivation of the enzyme.
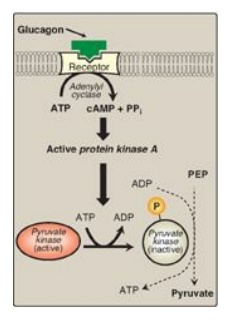
Figure 8.19 Covalent modification of hepatic pyruvate kinase results in inactivation of the enzyme. cAMP = cyclic AMP; PEP = phosphoenolpyruvate; P = phosphate; PPi = pyrophosphate.
3. Pyruvate kinase deficiency: Mature RBCs lack mitochondria and are, therefore, completely dependent on glycolysis for ATP production. ATP is required to meet the metabolic needs of RBCs and to fuel the ion pumps necessary for the maintenance of the flexible, biconcave shape that allows them to squeeze through narrow capillaries. The anemia observed in glycolytic enzyme deficiencies is a consequence of the reduced rate of glycolysis, leading to decreased ATP production. The resulting alterations in the RBC membrane lead to changes in cell shape and, ultimately, to phagocytosis by cells of the reticuloendothelial system, particularly macrophages of the spleen. The premature death and lysis of RBCs result in hemolytic anemia. Among patients exhibiting the rare genetic defects of glycolytic enzymes, the majority has a deficiency in PK. The effects of PK deficiency are restricted to RBCs and include mild-to-severe nonspherocytic hemolytic anemia, with the severe form requiring regular transfusions. [Note: Hepatic PK is encoded by the same gene as the RBC isozyme. Liver cells show no effect, however, because they have mitochondria and can generate ATP by oxidative phosphorylation.] Severity depends both on the degree of enzyme deficiency (generally 5–35% of normal levels) and on the extent to which RBCs compensate by synthesizing increased levels of 2,3-BPG. Almost all individuals with PK deficiency have a mutant enzyme that shows abnormal properties such as altered kinetics (Figure 8.20). Individuals heterozygous for PK deficiency have resistance to the most severe forms of malaria.
The tissue-specific expression of PK in RBCs and
the liver is the result of differential promoter utilization in transcription of
the gene that encodes both isozymes.
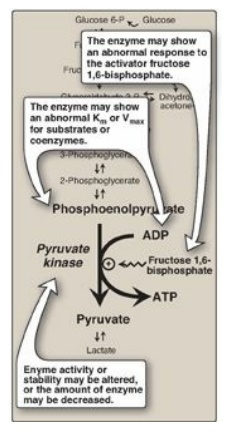
Figure 8.20 Alterations
observed with various mutant forms of pyruvate kinase. Km = Michaelis constant;
Vmax = maximal velocity.
K. Reduction of pyruvate to lactate
Lactate, formed by the action of lactate dehydrogenase, is the final product of anaerobic glycolysis in eukaryotic cells (Figure 8.21). The formation of lactate is the major fate for pyruvate in the lens and cornea of the eye, kidney medulla, testes, leukocytes, and RBCs, because these are all poorly vascularized and/or lack mitochondria.
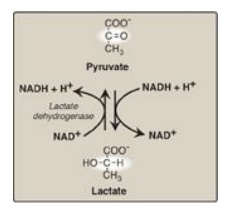
Figure 8.21 Interconversion of pyruvate and lactate. [Note: Lactate produced in muscle enters the circulation, is picked up by liver through facilitated diffusion, and is oxidized to pyruvate. Pyruvate is used by liver to make glucose.] NAD(H) = nicotinamide adenine dinucleotide.
1. Lactate formation in muscle: In exercising skeletal muscle, NADH
production (by glyceraldehyde 3-phosphate dehydrogenase and by the three NAD+-linked
dehydrogenases of the TCA cycle;) exceeds the oxidative capacity of the
respiratory chain. This results in an elevated NADH/NAD+ ratio,
favoring reduction of pyruvate to lactate. Therefore, during intense exercise,
lactate accumulates in muscle, causing a drop in the intracellular pH,
potentially resulting in cramps. Much of this lactate eventually diffuses into
the bloodstream and can be used by the liver to make glucose.
2. Lactate utilization: The direction of the lactate dehydrogenase reaction depends on the relative intracellular concentrations of pyruvate and lactate and on the ratio of NADH/NAD+ in the cell. For example, in the liver and heart, the ratio of NADH/NAD+ is lower than in exercising muscle. These tissues oxidize lactate (obtained from the blood) to pyruvate. In the liver, pyruvate is either converted to glucose by gluconeogenesis or oxidized in the TCA cycle. Heart muscle exclusively oxidizes lactate to CO2 and H2O via the TCA cycle.
3. Lactic acidosis: Elevated concentrations of lactate
in the plasma, termed lactic acidosis (a type of metabolic acidosis), occur
when there is a collapse of the circulatory system, such as in myocardial
infarction, pulmonary embolism, and uncontrolled hemorrhage, or when an
individual is in shock. The failure to bring adequate amounts of oxygen to the
tissues results in impaired oxidative phosphorylation and decreased ATP
synthesis. To survive, the cells rely on anaerobic glycolysis for generating
ATP, producing lactic acid as the end product. [Note: Production of even meager
amounts of ATP may be life-saving during the period required to reestablish
adequate blood flow to the tissues.] The excess oxygen required to recover from
a period when the availability of oxygen has been inadequate is termed the
“oxygen debt.”
The oxygen debt is often related to patient morbidity or mortality. In many clinical situations, measuring the blood levels of lactic acid allows the rapid, early detection of oxygen debt in patients and the monitoring of their recovery.
L. Energy yield from glycolysis
Despite the production
of some ATP during glycolysis, the end product, pyruvate or lactate, still
contains most of the energy originally contained in glucose. The TCA cycle is
required to release that energy completely.
1. Anaerobic glycolysis: Two molecules of ATP are generated
for each molecule of glucose converted to two molecules of lactate (Figure
8.22). There is no net production or consumption of NADH.
2. Aerobic glycolysis: The direct consumption and formation of ATP is the same as in anaerobic glycolysis (that is, a net gain of two ATP per molecule of glucose). Two molecules of NADH are also produced per molecule of glucose. Ongoing aerobic glycolysis requires the oxidation of most of this NADH by the electron transport chain, producing approximately three ATP for each NADH molecule entering the chain. [Note: NADH cannot cross the inner mitochondrial membrane, and substrate shuttles are required.]
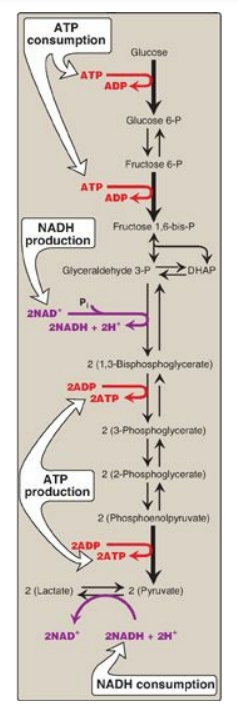
Figure 8.22 Summary of anaerobic glycolysis. Reactions involving the production or consumption of ATP or NADH are indicated. The three irreversible reactions of glycolysis are shown with thick arrows. DHAP = dihydroxyacetone phosphate; NAD(H) = nicotinamide adenine dinucleotide; P = phosphate.
Related Topics
