Regulation of Glycogenesis and Glycogenolysis
| Home | | Biochemistry |Chapter: Biochemistry : Glycogen Metabolism
Because of the importance of maintaining blood glucose levels, the synthesis and degradation of its glycogen storage form are tightly regulated.
REGULATION OF GLYCOGENESIS AND GLYCOGENOLYSIS
Because of the
importance of maintaining blood glucose levels, the synthesis and degradation
of its glycogen storage form are tightly regulated. In the liver, glycogenesis
accelerates during periods when the body has been well fed, whereas
glycogenolysis accelerates during periods of fasting. In skeletal muscle,
glycogenolysis occurs during active exercise, and glycogenesis begins as soon
as the muscle is again at rest. Regulation of glycogen synthesis and
degradation is accomplished on two levels. First, glycogen synthase and
glycogen phosphorylase are hormonally regulated (by phosphorylation/dephosphorylation)
to meet the needs of the body as a whole. [Note: Phosphorylation of glycogen
phosphorylase is catalyzed by glycogen phosphorylase kinase.] Second, these
same enzymes are allosterically regulated (by effector molecules) to meet the
needs of a particular tissue.
A. Activation of glycogen degradation
The binding of
hormones, such as glucagon or epinephrine, to plasma membrane G protein–coupled
receptors (GPCRs) signals the need for glycogen to be degraded, either to
elevate blood glucose levels or to provide energy for exercising muscle.
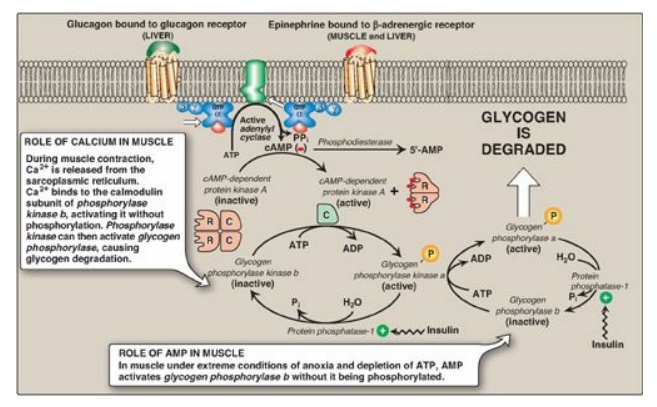
Figure 11.9 Stimulation and inhibition of glycogen degradation. AMP = adenosine monophosphate; cAMP = cyclic AMP; GTP = guanosine triphosphate; P = phosphate; PPi = pyrophosphate; R = regulatory subunit; C = catalytic subunit.
1. Activation of protein kinase A: Binding of glucagon or epinephrine
to their specific hepatocyte GPCR, or of epinephrine to a specific myocyte
GPCR, results in the G protein–mediated activation of adenylyl cyclase. This
enzyme catalyzes the synthesis of cyclic adenosine monophosphate (cAMP), which
activates cAMP-dependent protein kinase A (PKA), as described on page 95. PKA
is a tetramer, having two regulatory subunits (R) and two catalytic subunits
(C). cAMP binds to the regulatory subunits, releasing individual catalytic
subunits that are active (Figure 11.9). PKA then phosphorylates several enzymes
of glycogen metabolism, affecting their activity. [Note: When cAMP is removed,
the inactive tetramer, R 2C2, is again formed.]
2. Activation of phosphorylase kinase: Phosphorylase kinase exists in two
forms: an inactive “b” form and an active “a” form. Active PKA phosphorylates
the inactive “b” form of phosphorylase kinase, producing the active “a” form
(see Figure 11.9).
3. Activation of glycogen phosphorylase: Glycogen phosphorylase also exists
in two forms: the dephosphorylated, inactive “b” form and the phosphorylated,
active “a” form. Active phosphorylase kinase is the only enzyme that phosphorylates
glycogen phosphorylase b to its active “a” form, which then begins
glycogenolysis (see Figure 11.9).
4. Summary of the regulation of glycogen
degradation: The
cascade of reactions listed above results in glycogenolysis. The large number
of sequential steps serves to amplify the effect of the hormonal signal (that
is, a few hormone molecules binding to their receptors results in a number of
PKA molecules being activated that can each activate many phosphorylase kinase
molecules). This causes the production of many active glycogen phosphorylase a
molecules that can degrade glycogen.
5. Maintenance of the phosphorylated state: The phosphate groups added to phosphorylase kinase and phosphorylase in response to cAMP are maintained because the enzyme that hydrolytically removes the phosphate, protein phosphatase-1 (PP1), is inactivated by inhibitor proteins that are also phosphorylated and activated in response to cAMP (see Figure 11.9). [Note: PP1 is activated by a signal cascade initiated by insulin. Insulin also activates the phosphodiesterase that degrades cAMP, and, thus, insulin opposes the effects of glucagon and epinephrine.]
B. Inhibition of glycogen synthesis
The regulated enzyme in
glycogenesis is glycogen synthase. It also exists in two forms, the active “a”
form and the inactive “b” form. However, for glycogen synthase, in contrast to
phosphorylase kinase and phosphorylase, the active form is dephosphorylated,
whereas the inactive form is phosphorylated (Figure 11.10). Glycogen synthase a
is converted to the inactive “b” form by phosphorylation at several sites on
the enzyme, with the level of inactivation proportional to its degree of
phosphorylation. Phosphorylation is catalyzed by several different protein
kinases that are regulated by cAMP or other signaling mechanisms (see C.
below). Glycogen synthase b can be reconverted to the “a” form by PP1. Figure
11.11 summarizes the covalent regulation of glycogen metabolism.
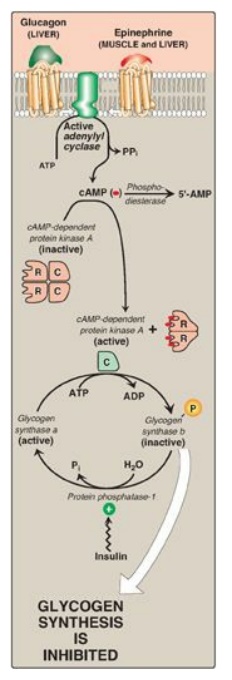
Figure 11.10 Hormonal regulation of glycogen synthesis. [Note: In contrast to glycogen phosphorylase, glycogen synthase is inactivated by phosphorylation.] cAMP = cyclic adenosine monophosphate; P = phosphate; PPi = pyrophosphate; R = regulatory subunit; C = catalytic subunit.
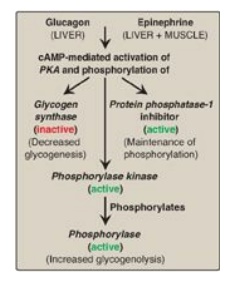
Figure 11.11 Summary of the
hormone-mediated covalent regulation of glycogen metabolism. cAMP = cyclic AMP;
PKA = protein kinase A.
C. Allosteric regulation of glycogen synthesis and degradation
In addition to hormonal
signals, glycogen synthase and glycogen phosphorylase respond to the levels of
metabolites and energy needs of the cell. Glycogenesis is stimulated when
substrate availability and energy levels are high, whereas glycogenolysis is
increased when glucose and energy levels are low. This allosteric regulation allows
a rapid response to the needs of a cell and can override the effects of
hormone-mediated covalent regulation.
1. Regulation of glycogen synthesis and degradation
in the well- fed state:
In the well-fed state,
glycogen synthase b in both liver and muscle is allosterically activated by
glucose 6-phosphate, which is present in elevated concentrations (Figure
11.12). In contrast, glycogen phosphorylase a is allosterically inhibited by
glucose 6-phosphate, as well as by ATP, a high-energy signal in the cell.
[Note: In liver, but not muscle, nonphosphorylated glucose is also an
allosteric inhibitor of glycogen phosphorylase a, making it a better substrate
for PP1.]
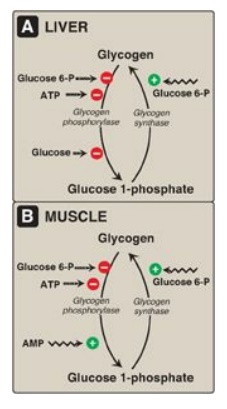
Figure 11.12 Allosteric regulation of glycogen synthesis and degradation. A. Liver. B. Muscle. P = phosphate.
2. Activation of glycogen degradation by calcium: Ca2+ is released into
the cytoplasm in muscle in response to neural stimulation and in liver in
response to epinephrine binding to α1-adrenergic receptors. The Ca2+
binds to calmodulin (CaM), the most widely distributed member of a family of
small, calcium-binding proteins. The binding of four molecules of Ca2+
to CaM triggers a conformational change such that the activated Ca2+–CaM
complex binds to and activates protein molecules, often enzymes, that are
inactive in the absence of this complex (see Figure 11.13). Thus, CaM functions
as an essential subunit of many complex proteins. One such protein is the
tetrameric phosphorylase kinase, whose b form is activated by the binding of Ca2+
to its δ subunit (CaM) without the need for the kinase to be phosphorylated by
PKA. [Note: Epinephrine at β-adrenergic receptors signals through rise in cAMP, not Ca2+.]
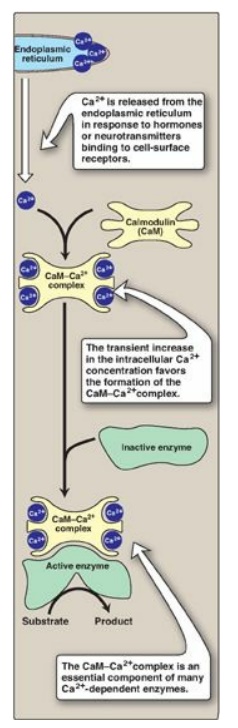
Figure 11.13 Calmodulin mediates many effects of intracellular Ca2+.
a. Calcium activation of muscle phosphorylase kinase: During muscle contraction, there is a rapid and urgent need for ATP. This energy is supplied by the degradation of muscle glycogen to glucose, which can then enter glycolysis. Nerve impulses cause membrane depolarization, which promotes Ca2+ release from the sarcoplasmic reticulum into the sarcoplasm of myocytes. The Ca2+ binds the CaM subunit, and the complex activates muscle phosphorylase kinase b (see Figure 11.9).
b. Calcium activation of liver phosphorylase
kinase:
During physiologic stress, epinephrine is released from the adrenal medulla and
signals the need for blood glucose. This glucose initially comes from hepatic
glycogenolysis. Binding of epinephrine to hepatocyte α-adrenergic GPCRs
activates a phospholipid-dependent cascade that results in movement of Ca2+
from the ER into the cytoplasm. A Ca2+–CaM complex forms and
activates hepatic phosphorylase kinase b. [Note: The released Ca2+
also helps to activate protein kinase C that can phosphorylate (therefore,
inactivate) glycogen synthase a.]
3. Activation of glycogen degradation in muscle: Muscle glycogen phosphorylase is
active in the presence of the high adenosine monophosphate (AMP) concentrations
that occur under extreme conditions of anoxia and ATP depletion. AMP binds to
glycogen phosphorylase b, causing its activation without phosphorylation (see
Figure 11.9). [Note: Recall that AMP also activates phosphofructokinase-1 of
glycolysis, allowing glucose from glycogenolysis to be oxidized.]
Related Topics
