Review questions answers
| Home | | Pharmaceutical Drugs and Dosage | | Pharmaceutical Industrial Management |Chapter: Pharmaceutical Drugs and Dosage: Chemical kinetics and stability
Pharmaceutical Drugs and Dosage: Chemical kinetics and stability - Review questions answers
Review questions
7.1 Use one
or more of the choices given below for answering the follow-ing set of
questions. These questions can be answered by referring to Figures
7.1 through 7.3.
i.
Zero-order reaction
ii. First-order reaction
iii. Second-order reaction
A. For which of these
reaction kinetic models is the drug con-centration at any time, t, independent of the initial drug
concentration?
B. For which of these
reaction kinetic models is the half-life inde-pendent of the initial drug
concentration?
C. For which of these
reaction kinetic models is the rate of drug degradation independent of the
initial drug concentration?
D. Which of these
reaction kinetic models would show an expo-nential decline in drug
concentration over a period of time?
E. Which of these
reaction kinetic models would give a straight line when concentration is
plotted against time?
F. Which of these
reaction kinetic models would give a straight line when the inverse of
concentration is plotted against time?
G. Which of these
reaction kinetic models would give a straight line when logarithm of
concentration is plotted against time?
H. For which of these
reaction kinetic models is the drug con-centration at any time t (and half-life) would be greater when
higher initial concentration is used (i.e., directly proportional to the
initial drug concentration)?
I.
For which of these reaction kinetic models is the drug
con-centration at any time t (and
half-life) would be lower when higher initial concentration is used (i.e.,
inversely proportional to the initial drug concentration)?
![]() 7.2 Which equation is used to predict the stability
of a drug at room tem-perature from experiments at increased temperatures?
7.2 Which equation is used to predict the stability
of a drug at room tem-perature from experiments at increased temperatures?
A. Stokes equation
B. Arrhenius equation
C. Michaelis–Menten
equation
D. Henderson–Hasselbalch
equation
E. Noyes–Whitney
equation
7.3 Which equation is
used to predict disproportionation of a weakly acidic or a weakly basic drug as
a function of pH?
A. Stokes equation
B. Arrhenius equation
C. Michaelis–Menten
equation
D. Henderson–Hasselbalch
equation
E. Noyes–Whitney
equation
7.4 When an
acid catalyzed reaction is affected by the concentration and strength of the buffer
species, it is known as:
A. Specific-acid
catalysis
B. Specific-base
catalysis
C. General-acid
catalysis
D. General-base
catalysis
7.5 In a
first-order reaction involving the decomposition of hydrogen per-oxide for a
period of 50 min, the concentration expressed in volume was found to be 10.6 mL
from an initial concentration of 72.6 mL.
A. Calculate k.
B. Calculate the amount
of hydrogen peroxide not decomposed after 30 min.
7.6 For a
second-order reaction,
C2H5COOC2H5
+ KOH → C2H5COOK + C2H5OH
Diethyl
acetate and potassium hydroxide were at a concentration of 0.05 M.
Concentration of potassium hydroxide was observed to change by 0.0088 mol/L
over a period of 35 min. Determine the rate constant, k, for the reaction and the half-life.
7.7 A
formulation for an analgesic is found to degrade at 110°C (383°K), with a rate constant of k1 = 2.0 h−1 and k2 at 150°C (383°K) of 3.8 h−1. Calculate the activation energy and the frequency
factor, A (R = 1.987 cal deg−1. mol−1).
7.8 The shelf
life of a liquid drug is 21 days at 5°C. For approximately how long will the drug be
stable at 37°C?
Answer:
7.1 A. Second order
B. First order
C. Zero order
D. First order
E. Zero order
F. Second order
G. First order
H. Zero and first order
I. Second order
7.2 B. Stability at room
temperature can be predicted from accelerated testing data by the Arrhenius
equation: log (k2/k1) = Ea (T2
− T1)/ (2.303 RT2T1), where k2
and k1 are the rate
constants at the abso-lute temperatures T2
and T1, respectively; R is the gas constant; and Ea is the energy of
activation. Stokes’ equation is used to deter-mine the sedimentation rate of a
suspension, whereas the Noyes– Whitney equation is used to determine the
dissolution rate.
7.3 I.
7.4 C.
7.5 A. 2H2O2
→ 2H2O + O2
For
afirst-order reaction,
k=
2.303/t log C0/C ,
k = 2 .303/50min * log (72.6mL/10.6mL)
= 0. 0385min−1
B. C = C010 − kt
/
2.303 = 72. 6 ∗ 10 −0.0385*30/ 2.303 = 22.885mL
7.6 To
calculate k, therefore diethyl
acetate and potassium hydroxide have same
initial concentration, x/a(a
− x) = kt, x = amount of reacted
potassium hydroxide, a = the initial
amount, ∴ k = x/(t * a * (a − x)) = 0.0088 mol/L/(35 min * 0.05
mol/L * (0.05 mol/L − 0.0088 mol/L)),
k = 0. 122 L, mol−1 min−1
To
calculate t1/2, i.e., x = 1/2a,
t = t1/ 2 = 1/2a/(ak(a. − 1/2a)) = 1/(ak) =
1/(0.05 mol/ L * 0.122 L mol−1 min−1) =
163.86 min
7.7
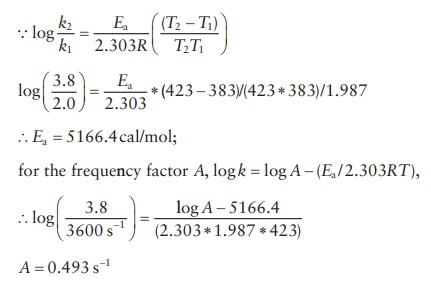
7.8 According
to t 90(T2 ) = t90(T1) /Q10ΔT /10, using Q10 of 5, life at 37°C = 21days/5[(37−5)/10] = 0.12
day or 2.92h
Related Topics
