SAR of Benzoic Acid Derivatives
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Local Anaesthetics
Most of these local anaesthetics are tertiary amines available as HCl salts with pKa in the range of 7.5–9.0.
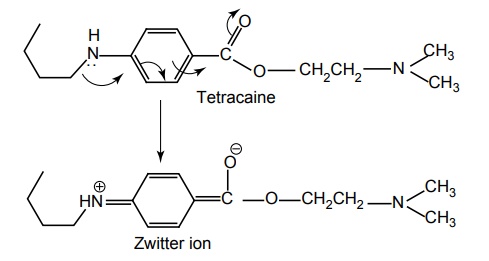
SAR OF BENZOIC ACID DERIVATIVES Most of these local anaesthetics are tertiary amines available as HCl salts with pKa in the range of 7.5–9.0. Any structural modification of the local anaesthetic that causes change in pKa will have pronounced effect to reach hypothetical receptor or the binding sites. The clinically useful local anaesthetics of this class possess an aryl radical that is attached directly to the carbonyl group and are highly liphophilic. They appear to play an important role in the binding of local anaesthetics to the channel receptor protein. Placement of aryl group with substituents that increases the electron density of the carbonyl oxygen enhances the activity. Structural modification leads to change in physical and chemical properties. Electron withdrawing substituents in ortho or para or at both the positions leads to an increase of its local anaesthetic property. Amino (procaine, butacaine) alkyl amino (tetracaine) alkoxyl (cyclomethycaine) group can contribute to electron density in the aromatic ring by both resonance and inductive effects. Hence the increase in local anaesthetic property. Any substitution that enhances zwitterion formation will be more potent. Hence m-position substitution decreases the activity. Tetracaine is more potent than procaine (40–50 times). Although the butyl group present in it increases lipid solubility, the potentiation is partly due to electron releasing property of the n-butyl group via inductive effect, which intend to increase the formation of the Zwitterion. Presence of electron withdrawing group such as C1– ortho to carbonyl pulls electron density away from carbonyl group, thus, making it more susceptible for nucleophilic attack by the esterase. In procaine series, anaesthetic potency decreases in the following order sulphur, oxygen, carbon, and nitrogen. Modifications also affect the duration of action and toxicity. In general, amides (X= N) are more resistant to metabolic hydrolysis than esters (X = O). Thioesters (X = S) may cause dermatitis. Placement of small alkyl groups (branching) around ester group (hexylcaine/meprylcaine) or the amide function also hinder hydrolysis, and hence, increase in duration of action. The amino alkyl group is not necessary for local anaesthetic activity, but it is used to form water soluble salts such as HCl salts. Tertiary amines are more useful agents. The secondary amines appear to have a longer duration of action, but they are more irritating. Primary amines are not active/cause irritation. The tertiary amino group may be diethyl amino, piperidine, or pyrolidino, leading to a product that exhibit same degree of activity, essentially. The more hydrophilic morpholino group usually leads to diminished potency. In general, the local anaesthetic drug should have increased lipid solubility and lower pKa values that leads to rapid onset and lower toxicity.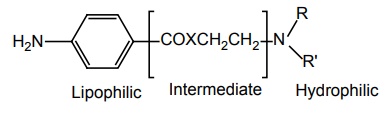
1. Lipophilic
2. Intermediate
3. Hydrophilic portion
Related Topics
