SAR of Dibenzo Cycloheptane Derivatives
| Home | | Medicinal Chemistry |Chapter: Medicinal Chemistry : Antidepressants
Placement of –Cl at C-3 shows increase in activity, whereas, CH3 at C-3 decreases CNS depression.
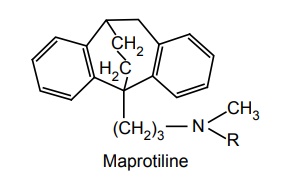
SAR of Dibenzo Cycloheptane Derivatives Placement of –Cl at C-3 shows increase in activity, whereas, CH3 at C-3 decreases CNS depression. Removal of N-methyl group of amitriptyline yielded nortriptyline, which is 2–5 times more potent than amitriptyline. Presence of double bond between 10 and 11 positions increases the activity. Unsaturation at C-5 enhances activity. When central ring size increases from 7 to 8 members (cyclo-octane), it is more effective. Several amitriptyline analogues in which replacement of C-11 with O, S, SO, SO2 , and NH are clinically effective antidepressants. Nortriptyline with exocyclic double bond and protriptyline with endocyclic double bond differ in their metabolism patterns. Protriptyline is less metabolized in vitro leading to a prolonged half-life and lower dose requirement. Novel bridged derivatives are very powerful, for example, maprotiline is a potent antidepressant, and the time to steady-state concentration is up to 7 days.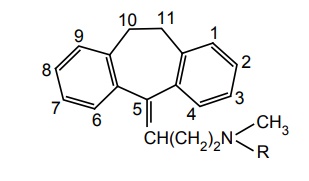
Related Topics
