Second-Order Splitting
| Home | | Organic Chemistry |Chapter: Organic Chemistry : Structure Determination of Organic Compounds
Our discussions of spin – spin splitting and multiplicity have been based on first-order or weakly coupled spectra which are spin systems where Δv/J ≥ 10.
SECOND-ORDER SPLITTING
Our
discussions of spin – spin splitting and multiplicity have been based on first-order or weakly coupled spectra which
are spin systems where Δv/J ≥ 10. The difference in chemical shift in hertz
of coupled protons divided by the coupling constant is 10 or more. In such a
case clean 1 : 1 doublets, 1 : 2 : 1 triplets, and so on, are observed and
coupling constants and chemical shifts can be read directly from line positions
in the spectrum.
As
Δv/J decreases, the simple multiplets
observed in weakly coupled spectra become increasingly distorted; new lines can
appear and others merge or disap-pear. Such spectra are termed second-order or strongly coupled spectra.
In these cases the chemical shift does not lie in the center of the multiplet
and coupling constants are not always obvious. A simple example of such a
change is seen
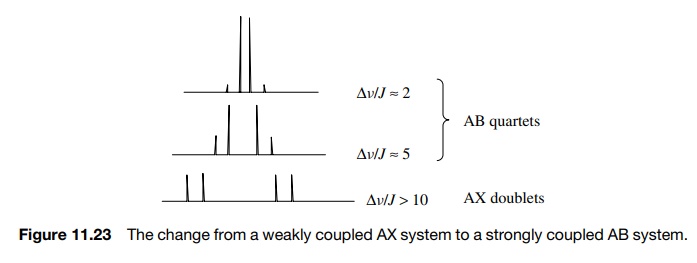
This is not a 1 : 3 :
3 : 1 quartet but an AB quartet in which the intensities of the inner and outer
lines depend on the difference in chemical shifts.
The
treatment of such systems is outside the scope of this book, but it is possible
to calculate the chemical shifts and coupling constants from line positions and
intensities. There are also experimental methods by which chemical shifts and
coupling constants can be determined in complex spectra. These include isotope
exchange, decoupling techniques, lanthanide shift reagents, and the use of
higher field NMR spectrometers. Since increases with the strength of the
magnetic field while J values do not
change with magnetic field strength, the ratio Δv/J increases as the field strength increases. Thus the higher the
field strength, the larger is the ratio Δv/J
and the greater is the chance to observe first-order coupling. In recent times
spectrometers of 300 – 500 MHz are routinely accessible so that the problems of
second-order spectra are becoming much less common. With the advent of even
higher field instruments, first-order spectra will be available for most
compounds. (The first commercial 750-MHz spectrometer was delivered in 1994.)
Related Topics
