Solid-Liquid interface
| Home | | Pharmaceutical Drugs and Dosage | | Pharmaceutical Industrial Management |Chapter: Pharmaceutical Drugs and Dosage: Interfacial phenomena
Many pharmaceutical systems deal with the adsorption of solutes from solutions onto solid surfaces.
Solid–Liquid
interface
Many
pharmaceutical systems deal with the adsorption of solutes from solutions onto
solid surfaces. These can be exemplified by the adsorption of drug or
hydrophilic polymer on suspended drug particles in a suspension or the
adsorption of drug on activated charcoal administered in the case of oral drug
overdose.
Modeling solute adsorption
The
adsorption of solute molecules from solution may be treated in a man-ner
analogous to the adsorption of gas molecules on the solid surface. Isothermal
adsorption can be expressed by Langmuir equation in the following form:
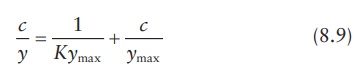
where,
c is the equilibrium concentration of
the solute in the solution and replaces p,
the partial pressure of the gas. A plot of c/y
against c yields a straight line, and
ymax and K can be obtained from the slope and
intercept of this plot.
The
Langmuir binding isotherm was utilized in determining the affinity and extent
of interaction of drug and excipients in the dosage form and the impact of this
interaction on oral bioavailability of drugs.
Factors affecting adsorption from solution
Adsorption
from solution depends on the following factors:
1. Solubility of
adsorbate/solute: The rate of adsorption of a solute is inversely proportional to its solubility in the solvent from
which adsorption occurs. For adsorption to occur, solute–solvent bonds must
first be broken. The greater the solubility, the stronger are these bonds and,
hence, the lower the rate of adsorption. Conversely, the lower the solubility
of the solute in the solvent, the higher its rate of adsorption onto the solid
adsorbent.
2. Solute
concentration:
An increase in the solute concentration increases the rate of adsorption that occurs at equilibrium until a limiting
value is reached.
3. Temperature: Adsorption is an
exothermic process, that is, heat is released
when stronger adsorbate–adsorbent bonds are formed. Thus, increase in
temperature reduces adsorption. This can also be under-stood as increased
Brownian motion of the solute molecules at higher temperature.
4. pH: The pKa value(s) of the solute determines the relative proportion of ionized and unionized species of the
solute and solute solubility in solution as a function of pH. The pH of the
solution may also influence surface polarity of the solid substrate by changing
the ion-ization, ion adsorption, or selective dissolution, as discussed
earlier. The effect of pH on adsorption depends on the nature of
intermolec-ular forces between solute and solute, solute and solvent, and
solute and solid substrate as a function of the ionization status of an ioniz-able
solute. The pH of the solution would also affect the solubility of the solute.
Adsorption
generally increases as the ionization of the drug is sup-pressed; that is, the
extent of adsorption reaches a maximum when the drug is completely unionized.
This is related to higher aqueous solubility of the ionized form. For
amphoteric compounds, adsorp-tion is at a maximum at the isoelectric point.
5. Nature of
adsorbent/solid substrate: The physicochemical nature of the adsorbent can affect the rate and extent of adsorption by
changes in the molecular forces of attraction between the adsorbate and the
adsorbent. In addition, the extent of adsorption is proportional to the surface
area of the adsorbent. Thus, an increased surface area, achieved by a reduction
in particle size or the use of a finely divide or porous adsorbing material,
increases the extent of adsorption.
Wettability and wetting agents
Adsorption
of the solvent, water, onto a solid substrate is termed wetting. The wettability of a material can be
ascertained by observing the contact angle that water makes with the surface. Contact angle is the angle between a
liquid droplet and the surface of the solid over which it spreads. As shown in Figure 8.5, the lower the contact angle (θ), the higher the wetting. Contact
angle can range from 0° to 180°. For example, mercury does not wet most solid
surfaces and its contact angle is well above 120° for most surfaces.
The
balance of intermolecular forces involved in determining the adsorp-tion of
solute on a solid surface is the same for the adsorption/wetting of
solvent/water on a solid surface. Powders, such as sulfur, charcoal, and
magnesium stearate, that are not easily wetted by water are called hydro-phobic. Powders, such as zinc
oxide, talc, and magnesium carbonate, that
are readily wetted by water are called hydrophilic.
A
wetting agent lowers the contact
angle and aids in displacing an air phase at the surface and replacing it with
a liquid phase. Wetting agents could be of the following types:
1. Surfactants: Surfactants with
hydrophile-lipophile balance (HLB) values
between 7 and 9 are used as wetting agents, generally in the concen-tration
of about 0.1% w/v. Surfactants reduce the interfacial tension between solid
particles and a vehicle. As a result of the lowered inter-facial tension, air
is displaced from the surface of particles, and wetting and deflocculation of
dispersed solid particles are promoted. Examples of surfactants used as wetting
agents include polysorbates (Tweens) and sorbitan esters (Spans), as well as
sodium lauryl sulfate.
2. Hydrophilic
colloids:
Acacia, bentonite, tragacanth, alginates, and cel-lulose derivatives act as
protective colloids by coating hydrophobic particles with a multimolecular
layer. This changes the surface proper-ties of the solid, making it more
hydrophilic, and promotes wetting.
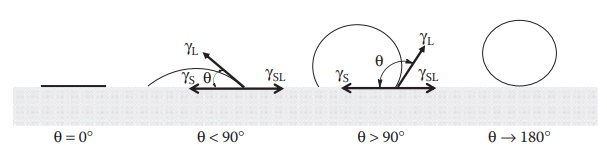
Figure 8.5 Contact angles from 0° to 180°.
3. Solvents: Water-miscible solvents, such as alcohol, glycerol, and glycols, can act as wetting agents by getting adsorbed on the solid surface, which makes the surface more hydrophilic, and reducing the dielectric constant of water, which can alter the balance of solute solu-bility in the bulk of the solvent versus surface adsorption.
Related Topics
